Rutherfordin
| Rutherfordin | |
|---|---|
| Rutherfordin from the Musonoi Mine , Kolwezi , Democratic Republic of the Congo (field of view 7 mm) | |
| General and classification | |
| other names |
Diderichite |
| chemical formula |
|
|
Mineral class (and possibly department) |
Carbonates and nitrates |
|
System no. to Strunz and to Dana |
5.EB.05 ( 8th edition : V / F.01) 01/14/04/01 |
| Crystallographic Data | |
| Crystal system | orthorhombic |
| Crystal class ; symbol | orthorhombic-dipyramidal; 2 / m 2 / m 2 / m |
| Room group (no.) | Imm 2 (No. 44) |
| Lattice parameters | a = 4.84 Å ; b = 9.27 Å; c = 4.30 Å |
| Formula units | Z = 2 |
| Physical Properties | |
| Mohs hardness | 2 to 3 |
| Density (g / cm 3 ) | measured: 5.7; calculated: 5.682 |
| Cleavage | completely after {010}, almost completely after {001} |
| colour | white, light yellow, straw yellow, greenish yellow, orange, amber brown |
| Line color | White |
| transparency | translucent |
| shine | matt or earthy, silk gloss in fibrous aggregates |
| Crystal optics | |
| Refractive indices |
n α = 1.700 to 1.723 n β = 1.716 to 1.730 n γ = 1.755 to 1.795 |
| Birefringence | δ = 0.055 to 0.072 |
| Axis angle | 2V = 53 ° (calculated) |
| Pleochroism | visible: X = colorless, Y = light yellow, Z = light greenish yellow |
Rutherfordin is a rarely occurring mineral from the mineral class of " carbonates and nitrates " (formerly carbonates, nitrates and borates, see classification ). It crystallizes in the orthorhombic crystal system with the composition (UO 2 ) (CO 3 ), so it is chemically a uranyl carbonate .
Rutherfordin is usually found in the form of dense to powdery masses on uraninite (UO 2 ), but also forms radial, fibrous to felt-like aggregates and, rarely, strip-shaped crystals up to about three millimeters in size. Its color varies from light yellow to almost white, straw yellow to greenish yellow or orange to amber brown.
Etymology and history
Rutherfordin was discovered on the western slope of Lukwengule in the Ulugurugebirge in Tanzania and was first described in 1906 by the German chemist Willy Marckwald (1864–1942), who named the mineral after the well-known atomic physicist Ernest Rutherford to honor his contribution to research into radioactivity. There are two type minerals, one of which is in the Natural History Museum Paris (Catalog No. 109.1083) and another at the National Museum of Natural History (Catalog No. 93291), Washington, DC, USA.
classification
In the now outdated, but still in use 8th edition of the mineral classification according to Strunz , rutherfordin belonged to the common class of carbonates, nitrates and borates and there to the division of "uranyl carbonates [UO 2 ] 2+ to [CO 3 ] 2− ", where together with blatonite , joliotite and oswaldpeetersite it formed the unnamed group V / F.01 .
The 9th edition of Strunz's mineral systematics , which has been in effect since 2001 and is used by the International Mineralogical Association (IMA), assigns rutherfordin to the abbreviated class “carbonates and nitrates”, but also to the category of “uranyl carbonates”. However, this is further subdivided according to the molar ratio of uranyl to carbonate complex, so that the mineral can be found according to its composition in the sub-section "UO 2 : CO 3 <1: 1 - 1: 2", where it is the only member of the unnamed group 5.EB.05 forms.
The systematics of minerals according to Dana , which is mainly used in the English-speaking world , assigns Rutherfordin, like the outdated Strunz system, to the common class of “carbonates, nitrates and borates” and there to the department of “anhydrous carbonates”. Here he is to be found as the only member / together with in the unnamed group 01/14/04 within the sub-section "Anhydrous carbonates with simple formula A + CO 3 ".
Crystal structure
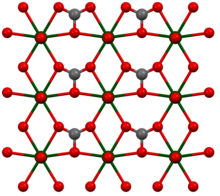
Rutherfordin crystallizes orthorhombically in the space group Imm 2 (space group no. 44) with the lattice parameters a = 4.84 Å ; b = 9.27 Å and c = 4.30 Å as well as two formula units per unit cell . The uranyl ion has a distorted hexagonal-bipyramidal structure . In the figure opposite, the uranyl oxygen atoms protrude upwards and downwards from the plane. In the equatorial plane , a carbonate anion coordinates four uranyl ions, so that they are linked to form linear layers. These layers lie parallel to one another in the crystal lattice, in such a way that the uranyl oxygen atoms coordinate the free coordination point of the carbonate anion, so that a slightly distorted trigonal-bipyramidal structure results for it.
properties
The mineral is radioactive due to its uranium content of up to 72.12% by weight . Taking into account the proportions of radioactive elements in the idealized empirical formula as well as the subsequent decays of the natural decay series, a specific activity of about 129.1 k Bq / g is given for the mineral (for comparison: natural potassium 0.0312 kBq / g). The quoted value can vary significantly depending on the mineral content and the composition of the levels; selective enrichment or depletion of the radioactive decay products is also possible and changes the activity.
In 1954, Hans W. Bültemann carried out fluorescence- analytical investigations on secondary uranium minerals, which is said to have included a strongly yellow-green fluorescent rutherfordin from the Morogoro region (Tanzania). Bültemann's observation could not be confirmed by synthetically produced and therefore pure rutherfordin. Fluorescence , which is rather improbable due to the composition, would only be the result of foreign admixtures and secondary mineral formation in naturally formed rutherfordin samples.
Education and Locations

As a typical secondary mineral, rutherfordin is formed by weathering from uraninite. In addition to this can be used as additional assemblages under a different Becquerelit , Billietit , Boltwoodit , Curit , Fourmarierit , Kasolite , Masuyit , Metatorbernit , Schoepit , Sklodowskit , studtite and Vanden Dries log occur.
As a rare mineral formation, rutherfordin could only be detected at a few sites, with around 50 sites being known to date (as of 2013). Its type locality Lukwengule in the Uluguru Mountains is the only known site in Tanzania so far .
In Germany, the mineral occurred in the pits "Sophia" near Wittichen , "Blessing God" near Schnellingen / Haslach in the Kinzig valley or Alpirsbach and " Krunkelbach " in Baden-Württemberg; in the "Johannessschacht" mine near Wölsendorf in Bavaria; in the uranium deposit Ellweiler in Rhineland-Palatinate and at Schneeberg in the Saxon Ore Mountains.
The only known site in Austria so far is the Übelskogel near Waldenstein (Wolfsberg municipality) in Carinthia, where the mineral was discovered in rock samples during the tunnel construction for the south A2 motorway.
Other locations include Australia, Brazil, China, the Democratic Republic of the Congo (Zaire) , France, Canada, Norway, the Czech Republic, the United Kingdom (Great Britain) and the United States of America (USA).
Rutherfordin (dark beige), billietite (orange) and uranophane (yellow) from the Shinkolobwe mine , Katanga , Democratic Republic of the Congo (size: 2.5 cm × 2.0 cm × 0.7 cm)
Rutherfordin (yellowish-brown coating) on uraninite from the Uluguru Mountains , Tanzania (0.9 cm × 0.8 cm × 0.7 cm)
Schoepit - Rutherfordin pseudomorphism from the Musonoi Mine , Katanga, Democratic Republic of the Congo ( overall size : 3.5 cm × 3.1 cm × 2.5 cm)
Precautions
Due to the high level of radioactivity , mineral samples of rutherfordin should only be kept in dust- and radiation-tight containers, but especially never in living rooms, bedrooms or workrooms. Absorption into the body ( incorporation , ingestion ) should also be prevented in any case and, for safety, direct body contact should be avoided and face masks and gloves should be worn when handling the mineral.
See also
literature
- W. Marckwald: About uranium ores from German East Africa. In: Zentralblatt für Mineralogie, Geologie und Paläontologie. Year 1906, pp. 761–763 ( rruff.info PDF 381 kB).
- C. Frondel, R. Meyrowitz: Studies of uranium minerals (XIX): Rutherfordine, Diderichite, and Clarkeite. In: American Mineralogist. Volume 41, 1956, pp. 127-133 ( rruff.info PDF 435 kB).
- Hans Jürgen Rösler : Textbook of Mineralogy . 4th revised and expanded edition. German publishing house for basic industry (VEB), Leipzig 1987, ISBN 3-342-00288-3 , p. 717 .
- Friedrich Klockmann : Klockmann's textbook of mineralogy . Ed .: Paul Ramdohr , Hugo Strunz . 16th edition. Enke, Stuttgart 1978, ISBN 3-432-82986-8 , pp. 583 (first edition: 1891).
Web links
- Mineral Atlas: Rutherfordin (Wiki)
Individual evidence
- ↑ a b IMA / CNMNC List of Mineral Names (PDF 1.3 MB; February 2013).
- ↑ a b c d Hugo Strunz , Ernest H. Nickel: Strunz Mineralogical Tables . 9th edition. E. Schweizerbart'sche Verlagbuchhandlung (Nägele and Obermiller), Stuttgart 2001, ISBN 3-510-65188-X , p. 319 .
- ↑ a b Stefan Weiß: The large Lapis mineral directory. All minerals from A - Z and their properties . 5th, completely reworked and supplemented edition. Weise, Munich 2008, ISBN 978-3-921656-70-9 .
- ↑ a b Rutherfordine. In: John W. Anthony, Richard A. Bideaux, Kenneth W. Bladh, Monte C. Nichols (Eds.): Handbook of Mineralogy, Mineralogical Society of America. 2001 ( handbookofmineralogy.org PDF 65.6 kB).
- ↑ a b c d Mindat - Rutherfordine.
- ↑ "W. Marckwald, Ueber uranium ores from German East Africa, Zentralbl. Min., Geol. Paläont., 1906, 761-63. "
- ^ RJ Finch, MA Cooper, FC Hawthorne, RC Ewing: Refinement of the crystal structure of rutherfordine. In: The Canadian Mineralogist. Volume 37, 1999, pp. 929-938 ( rruff.info PDF 870 kB).
- ↑ Webmineral - rutherfordine.
- ^ C. Frondel, R. Meyrowitz: Studies of uranium minerals (XIX): rutherfordine, diderichite, and clarkeite. In: American Mineralogist. Volume 41, 1956, p. 130 ( rruff.info PDF 435 kB).
- ↑ Mindat - Number of localities for Rutherfordin
- ↑ Find location lists for rutherfordin in the Mineralienatlas and Mindat .