Becquerelite
| Becquerelite | |
|---|---|
| Tufted Becquerelite from the Shinkolobwe Mine , Katanga , Democratic Republic of the Congo (length of the crystals: 5 mm) | |
| General and classification | |
| chemical formula | Ca [(UO 2 ) 6 | O 4 | (OH) 6 ] • 8H 2 O |
|
Mineral class (and possibly department) |
Oxides and hydroxides |
|
System no. to Strunz and to Dana |
4.GB.10 ( 8th edition : IV / H.03) 07/05/01/02 |
| Crystallographic Data | |
| Crystal system | orthorhombic |
| Crystal class ; symbol | orthorhombic-pyramidal, mm2 |
| Room group (no.) | Pn 2 1 a (No. 33) |
| Lattice parameters | a = 13.84 Å ; b = 12.38 Å; c = 14.92 Å |
| Formula units | Z = 4 |
| Physical Properties | |
| Mohs hardness | 2.5 |
| Density (g / cm 3 ) | measured: 5.09 to 5.2; calculated: 5.10 |
| Cleavage | completely after {001}; imperfect after {101}, {010} and {110} |
| colour | brown-yellow, amber-yellow to lemon-yellow, yellow-orange |
| Line color | yellow |
| transparency | transparent |
| shine | Diamond shine to greasy shine |
| radioactivity | very strong |
| Crystal optics | |
| Refractive indices |
n α = 1.725 to 1.735 n β = 1.815 to 1.825 n γ = 1.825 to 1.830 |
| Birefringence | δ = 0.100 |
| Optical character | biaxial negative |
| Axis angle | 2V = measured: 32 ° |
| Pleochroism | visible: X = colorless to light yellow, Y = Z = yellow to dark yellow |
| Other properties | |
| Special features | fluorescence |
Becquerelite is a rather seldom occurring mineral from the mineral class of " oxides and hydroxides ". It crystallizes in the orthorhombic crystal system with the chemical composition Ca [(UO 2 ) 6 | O 4 | (OH) 6 ] · 8H 2 O and develops mostly transparent, tabular to prismatic and pseudo-hexagonal crystals , but also granular aggregates and crusts of brownish yellow, Amber yellow to lemon yellow and yellow-orange color with yellow streak color . A diamond-like to grease-like shine appears on the crystal surfaces .
Etymology and history
Becquerelite was first discovered in the Shinkolobwe mine in the Haut-Katanga province of the Democratic Republic of the Congo and described in 1922 by Alfred Schoep (1881-1966), who named the mineral in honor of the discoverer of radioactivity, Antoine Henri Becquerel (1852-1908) .
classification
In the now outdated, but still in use 8th edition of the mineral classification according to Strunz , the Becquerelite belonged to the mineral class of "oxides and hydroxides" and there to the department of "uranyl hydroxides and hydrates", where together with billietite , compreignacite , masuyit and Protasit formed an independent group.
The 9th edition of Strunz's mineral systematics , which has been in effect since 2001 and is used by the International Mineralogical Association (IMA), also assigns Becquerelite to the class of "oxides and hydroxides" and there in the department of "uranyl hydroxides". However, this section is further subdivided according to the presence of further cations and the crystal structure, so that the mineral is classified according to its composition and structure in the sub-section “With additional cations (K, Ca, Ba, Pb etc.), with predominantly UO 2 (O , OH) 5 pentagonal polyhedra "is to be found, where it is named after the" Becquerelite group "with the system no. 4.GB.10 and the other members Billietit and Protasit.
The systematics of minerals according to Dana also assigns becquerelite to the class of "oxides and hydroxides", but there in the category of "uranium and thorium-containing oxides". Here he is also the namesake of the "Becquerelit Group" with the system no. 05.07.01 and the other members Compreignacit and Billietit can be found within the subsection of " Uranium and thorium-containing oxides with alkali or hydrated hydroxide components ".
Crystal structure
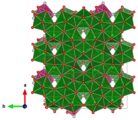
Becquerelite crystallizes orthorhombically in the space group Pn 2 1 a (space group no. 33) with the lattice parameters a = 13.84 Å ; b = 12.38 Å and c = 14.92 Å as well as 4 formula units per unit cell .
Packing pattern of Becquerelite in the direction of the crystallographic a -axis ( __ U __ O __ Ca __ water molecules )
Packing pattern of Becquerelite in the direction of the crystallographic b -axis ( __ U __ O __ Ca __ water molecules )
Packing image of Becquerelite in the direction of the crystallographic c- axis ( __ U __ O __ Ca __ water molecules )
The crystal structure of Becquerelite consists of layers of edge-linked pentagonal-bipyramidal uranyl units, which are bridged to one another by Ca 2+ ions, which are also coordinated by four water of crystallization molecules. Four other water molecules are held in the structure by hydrogen bonds alone . The OH groups connect the uranium atoms equatorially in the layers; their hydrogen atoms are also coordinated by the free crystal water molecules via hydrogen bonds.
properties
The mineral is radioactive due to its uranium content of up to 72% . Taking into account the proportions of the radioactive elements in the idealized empirical formula and the subsequent decays of the natural decay series, a specific activity of about 129.7 k Bq / g is specified for the mineral (for comparison: natural potassium 0.0312 kBq / g). The quoted value can vary significantly depending on the mineral content and the composition of the levels; selective enrichment or depletion of the radioactive decay products is also possible and changes the activity.
Education and Locations

Becquerelite is secondary to the weathering of uraninite in the oxidized parts of uranium deposits, but rarely also in the pegmatites . In addition to uraninite, accompanying minerals include schoepite , soddyite , curite , fourmarierite , dewindtite , ianthinite , wölsendorfite , rutherfordine , masuyite , kasolite , johannite , uranopilite and zippeit .
Peter Burns et al. describe Becquerelite as a conversion product of spent nuclear fuel . They were also able to show that synthetic becquerelite crystals, which are structurally identical to natural becquerelite, can exchange calcium ions for strontium ions by heating the crystals in a SrCl 2 solution at 160 ° C for 50 hours. The structure is retained, but the dimensions of the unit cell and the space group change . In analogy to the cation exchange in boltwoodite ( Cs for K and Na ) already demonstrated by the authors , this experiment shows the principle possibility of ion exchange in secondary uranium minerals, which are of geological and ecological importance for the storage of radiotoxicologically relevant nuclides ( 137 Cs , 90 Sr ) is. Nevertheless, the authors admit that it is not entirely clear whether these processes can also take place under the geological conditions of a repository or in realistic solutions with ion concentrations between 100 and 1000 ppm . Appropriate experiments to examine this problem more closely are still pending.
So far (as of 2014), Becquerelite has been detected at around 90 sites worldwide. In addition to its type locality Shinkolobwe Mine , the mineral was also found in the Democratic Republic of the Congo at Kolwezi in the Kamoto Principal Mine and the Musonoi Mine .
In Germany Becquerelit appeared in the Clara mine in Baden-Württemberg; near Wölsendorf in the Schwandorf district in Bavaria; in the uranium deposit of Ellweiler in Rhineland-Palatinate and at Johanngeorgenstadt and Tirpersdorf in Saxony. In Austria, the mineral has so far only been found near Mitterberg in the municipality of Mühlbach am Hochkönig (Salzburg) and in Switzerland it was found in several areas of the Trient Valley and near Isérables in the canton of Valais.
Other locations are Argentina , Australia , China , England (United Kingdom), France , Italy , Canada , Madagascar , Mexico , New Zealand , Norway , Romania , Russia , South Africa , the Czech Republic and the USA .
Precautions
Due to the toxicity and the strong radioactivity of the mineral, mineral samples from Becquerelite should only be kept in dust- and radiation-proof containers, but especially never in living rooms, bedrooms or work rooms. Absorption into the body (incorporation, ingestion ) should also be prevented in any case and, for safety, direct body contact should be avoided and respiratory protection mask and gloves should be worn when handling the mineral .
See also
Individual evidence
- ↑ a b c d e Hugo Strunz , Ernest H. Nickel: Strunz Mineralogical Tables . 9th edition. E. Schweizerbart'sche Verlagbuchhandlung (Nägele and Obermiller), Stuttgart 2001, ISBN 3-510-65188-X , p. 251 .
- ↑ a b Webmineral - Becquerelite (English)
- ↑ Handbook of Mineralogy - Becquerelite (English, PDF 72.8 kB)
- ↑ a b Mindat - Becquerelite (English)
- ↑ a b PC Burns, Y. Li: The structures of becquerelite and Sr-exchanged becquerelite In: American Mineralogist 2002, 87, pp. 550-557. (English, PDF, 1.4 MB)
literature
- Paul Ramdohr , Hugo Strunz : Klockmann's textbook of mineralogy . 16th edition. Ferdinand Enke Verlag, 1978, ISBN 3-432-82986-8 , pp. 559 .