Uranopilite
| Uranopilite | |
|---|---|
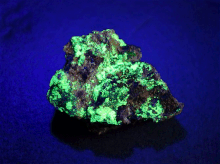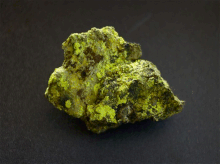
| Yellow uranopilite on coffinite and pitchblende ( uraninite ) from Les Mares III, Lodève, Occitania , Département Hérault , France | |
| General and classification | |
| other names |
|
| chemical formula |
|
|
Mineral class (and possibly department) |
Sulphates (selenates, tellurates, chromates, molybdates, wolframates) (formerly sulfates, chromates, molybdates and tungstates) |
|
System no. to Strunz and to Dana |
7.EA.05 ( 8th edition : VI / D.20) 02/31/06/01 |
| Crystallographic Data | |
| Crystal system | triclinic |
| Crystal class ; symbol | triclinic pinacoidal; 1 |
| Space group | P 1 (No. 2) |
| Lattice parameters |
a = 8.896 Å ; b = 14.029 Å; c = 14.339 Å α = 96.610 °; β = 98.472 °; γ = 99.802 ° |
| Formula units | Z = 2 |
| Physical Properties | |
| Mohs hardness | ~ 2 |
| Density (g / cm 3 ) | measured: 3.7 to 4.0; calculated: 4.045 |
| Cleavage | completely after {010} |
| colour | light yellow, lemon yellow, golden yellow, straw yellow |
| Line color | Please complete |
| transparency | translucent |
| shine | Silky gloss |
| radioactivity | 121.59 kBq / g |
| Crystal optics | |
| Refractive indices |
n α = 1.621 to 1.623 n β = 1.623 to 1.625 n γ = 1.632 to 1.634 |
| Birefringence | δ = 0.011 |
| Optical character | biaxial positive |
| Axis angle | 2V = 50 ° (measured), 52 ° (calculated) |
| Pleochroism | x = colorless; y and z = yellow |
| Other properties | |
| Chemical behavior | soluble in water and dilute acids |
Uranopilit is a frequently occurring mineral from the mineral class of sulfates, selenates, tellurates, chromates, molybdates and tungstates (formerly sulfates, chromates, molybdates and tungstates) with the chemical composition [(UO 2 ) 6 (SO 4 ) O 2 (OH) 6 ] · 14H 2 O. The content of water of crystallization can fluctuate between 12 and 14 molecules of H 2 O and drops in dry air to a content of 5 molecules per formula unit.
Uranopilit crystallizes in the triclinic crystal system , but develops only microscopic, needle-like crystals up to 0.1 mm in size. Usually these grow together to form spherical or felt-like mineral aggregates as well as crusty coatings and efflorescence on a uraninite matrix. The crystals are translucent and light yellow, lemon yellow, golden yellow or straw yellow in color.
Etymology and history

Investigations into the chemical and crystallographic structure of naturally occurring uranyl sulfates have always extended over a long period of time, also because the minerals were mostly only found as fine, contaminated powder-like coatings. Already Albin Weisbach (1833–1901) noted in the first mention of the mineral in the "New Yearbook for Mineralogy and Paleontology" in 1882:
"This name [Uranocher] is used to designate various bodies, which are partly [sic!] Uranium oxide hydrates, partly also uranium hydrosulfates, the knowledge of which leaves a lot to be desired in physical terms."
He also suggested naming it Uranopilit. One of the first investigations of a mineral stage with the formula (UO 2 ) 6 SO 4 (OH) 10 O 2 · 5H 2 O is that of Radim Nováček (1905–1942) in 1942. He assumed it was partially dehydrated uranopilite and described the mineral as β-uranopilite. Clifford Frondel later renamed this meta-uranopilite in his investigations. Based on this investigation in 1958, the chemical formula (UO 2 ) 6 (SO 4 ) (OH) 10 · 12H 2 O is now considered to be generally applicable.
The mineral was named after its uranium content and the Greek word πίλος [pronounced: "pilos"] for felt, which means "uranium felt". The type locality is the “Georg Wagsfort” mine near Johanngeorgenstadt in the Saxon Ore Mountains in Germany .
classification
In the outdated, but partly still in use 8th edition of the mineral classification according to Strunz , the uranopilite belonged to the common mineral class of "sulfates, selenates, tellurates, chromates, molybdates and tungstates" and there to the department of "water-containing sulfates with foreign anions" where it belongs together with Cobaltzippeit , Jáchymovit , Natrozippeit , Magnesiumzippeit , Marécottit , Metauranopilit , Nickelzippeit , Rabejacit , Zinkzippeit and Zippeit the Uranopilite group VI / D.20 formed.
The 9th edition of Strunz's mineral systematics , which has been in effect since 2001 and is used by the International Mineralogical Association (IMA), assigns uranopilite to the class of "sulfates, selenates, tellurates, chromates, molybdates and wolframates", but in the department of "Uranyl Sulphate". This is further subdivided so that the mineral can be found according to its composition in the sub-section "without cations", where it is located as a member of the uranopilite group 7.EA.05 with the metauranopilite .
The systematics of minerals according to Dana , which is predominantly used in the English-speaking world , assigns uranopilite to the class of "sulfates, chromates and molybdates" and there to the category of "water-containing sulfates with hydroxyl or halogen". Here it can be found together with Jáchymovite in the unnamed group 02/31/06 within the subsection “ Hydrogen sulfates with hydroxyl or halogen with (A + B 2+ ) 6 (XO 4 ) Z q × x (H 2 O)”.
Crystal structure
Uranopilit crystallizes triclinically in the space group P 1 (space group no. 2) with the lattice parameters a = 8.896 Å ; b = 14.029 Å; c = 14.339 Å; α = 96.610 °; β = 98.472 ° and γ = 99.802 ° as well as two formula units per unit cell .
properties
With its uranium content of up to 67.9%, the mineral is radioactive . Taking into account the proportions of radioactive elements in the idealized empirical formula and the subsequent decays of the natural decay series, a specific activity of 121.59 k Bq / g is specified for the mineral (for comparison: natural potassium 0.0312 kBq / g). The quoted value can vary significantly depending on the mineral content and the composition of the levels; selective enrichment or depletion of the radioactive decay products is also possible and changes the activity.
Uranopilit is readily soluble in water and dilute acids and shows a strong yellow-green fluorescence under UV light . Investigations have shown that dehydration takes place from a temperature of 31 ° C and metauranopilite is formed. Above 80 ° C a hydroxyl group is split off .
Education and Locations
Uranopilit forms in the area of acidic oxidation zones of sulphide deposits in the presence of uraninite . In addition to uraninite, accompanying minerals include fourmarierite , gypsum , Johannite , soddyite , uranophane and zippeit .
As a rare mineral formation, uranopilite could only be detected at a few sites, with around 90 sites so far (as of 2016) being known. In addition to its type locality Johanngeorgenstadt, the mineral appeared in Saxony in various places in the Ore Mountains such as Annaberg-Buchholz , Altenberg , Marienberg and the pits of the Schneeberg, Schlema and Alberoda district . Other sites in Germany are also in Baden-Württemberg (Müllenbach, Krunkelbach mine ), Bavaria ( Stulln ), Rhineland-Palatinate ( Ellweiler ), Saxony-Anhalt (Mansfeld Basin) and Thuringia ( Ronneburg ).
The only known site in Austria so far is the Hüttenberger Erzberg in the Seetal Alps in the north-east of Carinthia and the only site in Switzerland so far is the uranium prospectus La Creusaz near Les Marécottes (Trient Valley) in the canton of Valais .
Uranopilite was found worldwide in Argentina, Australia, China, France, Gabon, Italy, Japan, Canada, the Democratic Republic of the Congo, Poland, Slovakia, Slovenia, the Czech Republic, Hungary, the United Kingdom (UK) and the United States of America (UNITED STATES).
Particularly noteworthy are the sites in the Permian Basin of Lodève (southern France), which are coveted by collectors and produce specimens that are fluorescent even in daylight.
Precautions
Due to the toxicity and the strong radioactivity of the mineral, mineral samples from uranopilite should only be kept in dust- and radiation-proof containers, but especially never in living rooms, bedrooms or work rooms. Absorption into the body (incorporation, ingestion ) should also be prevented in any case and, for safety, direct body contact should be avoided and respiratory protection mask and gloves should be worn when handling the mineral .
See also
literature
- Albin Weisbach : Mineralogical Notes II: 17th Uranocher. In: New Yearbook for Mineralogy, Geology and Palaeontology Year 1882 (Volume 2), pp. 258–259 ( PDF 4.1 MB ).
- Radim Novácek: Study on some secondary uranium minerals (in English). In: Vestniku Královské Ceské Spolecnosti Nauk Volume 7 (1935), pp. 1–36 ( PDF 2.1 MB )
Web links
Individual evidence
- ^ A b Albin Weisbach : Mineralogical Notes II: 17. Uranocher. In: New Yearbook for Mineralogy, Geology and Palaeontology Year 1882 (Volume 2), pp. 258–259 ( PDF 4.1 MB ).
- ^ Helmut Schrätze , Karl-Ludwig Weiner : Mineralogie. A textbook on a systematic basis . de Gruyter, Berlin; New York 1981, ISBN 3-11-006823-0 , pp. 597 .
- ^ Hugo Strunz , Ernest H. Nickel : Strunz Mineralogical Tables. Chemical-structural Mineral Classification System . 9th edition. E. Schweizerbart'sche Verlagbuchhandlung (Nägele and Obermiller), Stuttgart 2001, ISBN 3-510-65188-X , p. 413 .
- ↑ a b c d Peter C. Burns: A new Uranyl Sulfate chain in the Structure of Uranopilite. In: Canadian Mineralogist. 2001, Volume 39, pp. 1139–1146 online (PDF 372 kB)
- ↑ a b IMA / CNMNC List of Mineral Names; May 2016 (PDF 1.6 MB)
- ↑ a b Ray L. Frost, Matt L. Weier, Godwin A. Ayoko, Wayde Martens, Jiří Čejka: An XRD, SEM and TG study of a uranopilite from Australia. ( PDF (English) 183 kB )
- ↑ a b c d Webmineral - Uranopilite
- ↑ a b American-Mineralogist-Crystal-Structure-Database - Uranopilite
- ↑ a b c d Uranopilite , In: John W. Anthony, Richard A. Bideaux, Kenneth W. Bladh, Monte C. Nichols (Eds.): Handbook of Mineralogy, Mineralogical Society of America , 2001 ( PDF, English 66.8 kB )
- ↑ a b c d e f Mindat - Uranopilite
- ↑ Hans Jürgen Rösler : Textbook of Mineralogy . 4th revised and expanded edition. German publishing house for basic industry (VEB), Leipzig 1987, ISBN 3-342-00288-3 , p. 418 .
- ^ Clifford Frondel : Studies on uranium minerals (IX): Saléeite and Nováčekite. In: American Mineralogist Volume 36 (1951), pp. 525-530 ( PDF 473 KB )
- ↑ Mindat - Number of localities for Uranopilit
- ↑ Find location list for uranopilite at the Mineralienatlas and at Mindat