Aramids
| General structure of aramids |
|
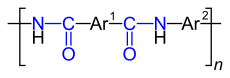
| Repeating units of aromatic polyamides made from a diamine and a dicarboxylic acid chloride. Ar 1 stands for the “ aryl radical ” of the dicarboxylic acid chloride used, Ar 2 for the aryl radical of the diamine compound used. The carboxamide groups are marked in blue . |
Aramids (a word summary of Ar omatische poly amide ) of the ISO is a generic term for such polyamides , in which the amide groups to aromatic groups are bonded. Aramids are among the liquid crystal polymers (FKP). They are mainly produced in the form of fibers (both filaments and staple fibers ), but also in the form of fibrids and pulps , foils , papers and particles. According to the definition of the US Federal Trade Commission for textile fibers, aramids are polyamides with aromatic groups in the main chain in which at least 85% of the amide groups are bonded directly to two aromatic rings. The European Textile Labeling Ordinance also requires this, but also designates aromatic polyamide-imides as aramids.
A distinction is made between meta -aramids, para -aramids and para -aramid copolymers . Among the high-performance fibers, m-aramid fibers belong to the group of infusible, high-temperature and flame-resistant fibers with mechanical properties in the area of conventional textile fibers. They are characterized by exceptional chemical resistance and high heat resistance. The p-aramid fibers as well as the p-aramid copolymer fibers belong to the group of high-strength synthetic fibers with increased temperature resistance. Well-known brand names for m-aramid fibers are Nomex from DuPont and Teijinconex from Tejin Aramid, for p-aramid fibers Kevlar from DuPont and Twaron from Teijin Aramid and for para-aramid copolymer Technora from Teijin Aramid.
 Poly ( p- phenylene terephthalamide) |
 Poly ( m -phenylene isophthalamide) |
History of the aramid
The search for high strength and high temperature resistant fibers has been triggered to a large extent by the need for space travel. As early as the 1940s, chemical fiber research recognized that polyamides containing an aromatic core had relatively high melting points and were more rigid and dimensionally stable than those with aliphatic groups. But it was also clear that high-melting, fully aromatic polyamides could not be spun from the melt and would also be sparingly soluble. Therefore, some technological innovations were required for the production of fully aromatic polyamides with high molecular weight.
In 1950, the chemist Emerson Wittbecker developed interfacial polycondensation at DuPont in the USA . He received evidence of this from the Allied report on German synthetic fiber research before World War II. The method was further developed at DuPont and expanded by Paul Morgan to include solution polycondensation. Based on these polycondensation methods, the researchers at DuPont invented the heat-resistant poly ( m -phenylene terephthalamide) fiber HT-1 in the late 1950s / early 1960s under the direction of PW Morgan , which was made from a filament as well as staple fiber and paper Mixture of short length staple fibers with HT-1 fibrids. The fiber was launched in 1962 under the trade name Nomex .
Another technological advance was achieved when in 1965 Stephanie Kwolek discovered the liquid-crystalline behavior of p-aramids in solution and also concentrated on the synthesis of poly ( p- phenylene terephthalamide), because this p-aramid was based on inexpensive starting materials and the development of a made completely new spinning processes possible by Herbert Blades. The first fiber made from p-aramid was called Fiber B and its manufacturing process was scaled up in 1971, so that the first production facility could be built with an annual capacity of approx. 2,000 t. It was launched under the brand name Kevlar in 1972
Also in the late 1950s, research on aramids began in the former Soviet Union , which was sponsored by the military. The fiber, which was comparable to the heat-resistant m-aramid fiber Nomex, was given the brand name Fenilon . It was first manufactured in 1969 on a pilot plant of the Union Institute for Synthetic Fiber Research, but it wasn't until 1985 that the first industrial production plant went into operation. A fiber with high strength and high modulus was also developed in 1969 under the name Vniivlon , the name of which was changed to SVM after further improvements and was available for further processing technologies from 1972. Later Armos, an aramid copolymer fiber, and a p-aramid fiber were produced.
The British company ICI also started a research program for the production of aramid fibers in the mid / late 1960s, also produced some fibers on a small-scale facility, but stopped work in 1976 due to a management decision.
In the early 1970s, the Dutch company AKZO also began developing aramid fibers. At the end of 1972, the AKZO researchers developed an aramid fiber with properties comparable to fiber B (Kevlar) from DuPont. AKZO developed this fiber as fiber X, from 1975 further as Arenka . In 1976 the pilot plant for this fiber was put into operation. 1978 began with the preparations of a large plant for aramid polymers and a spinning plant for fibers. Arenka was renamed Twaron in 1982 . Commercial production began in 1985. In 1989 AKZO's aramid business was spun off into a separate business unit, Twaron BV, which was taken over by the Japanese Tejjin Group in 2000. In 2007 the name Teijin Twaron BV became Teijin Aramid BV. In Japan, Tejjin started producing a heat-resistant aramid fiber as early as 1969, which was similar to Nomex . It was given the brand name Conex , now known as Teijinconex . Also, a high strength, high modulus aramid fiber was developed by Teijin in the 1970s, which was designated HM-50. In 1987 the first production facility was opened. The fiber was given the Technora brand name .
In 1979, Kolon Industries began developing p-aramid in the Republic of Korea . Today filaments, staple fibers and pulp are produced under the brand name Heracron .
In the People's Republic of China , Yantai Tayho Advanced Materials Co., Ltd produces m-aramid fibers under the brand name Newstar and p-aramid fibers under the Taparan brand.
synthesis
In the synthesis of the aramids, an aromatic di carboxylic acid halide and a phenylenediamine are assumed, for example. B. of paraphenylenediamine and terephthaloyl dichloride .
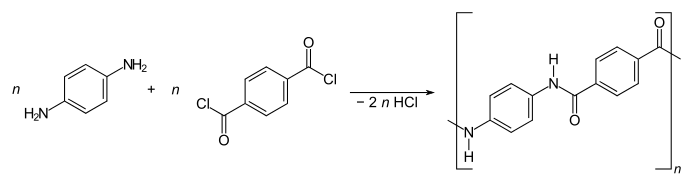
- Polycondensation in the production of PPTA
The synthesis takes place according to the Schotten-Baumann method at temperatures in the range from 0 to −40 ° C in order to avoid side reactions. N- methylpyrrolidone , dimethylacetamide or tetramethylurea to which salts such as calcium chloride are added are used as solvents .
be crazy
The processing into fibers can only take place from solutions, since the melting point is usually far above the thermal decomposition point. A high polymer concentration in the spinning solution is advantageous for filament production and can lead to high orientations. A good solvent for aramids in high concentration and thus anisotropic character is concentrated sulfuric acid . The direct spinning route from the polymer solution has not proven to be practicable; polymers of the para-oriented, aromatic dicarboxylic acids and diamines type are more economical . The fiber production via polycondensation and the use of sulfuric acid as a solvent is shown in the picture.
The spinning process is the usual classic wet spinning process. The use of an air gap between the spinneret and the spinning bath, as u. a. known from acrylic spinning has advantages. After drying, the yarn has a high strength and a high modulus of elasticity . In a second process stage, the yarn can be drawn at temperatures of 300 ° C to 400 ° C. This leads to an even higher modulus with the same strength and lower elongation at break. This type of aramid fiber is used in a wide variety of applications.
properties
Aramids have high tensile strength and are very tough, which results in a high energy absorption capacity; its tear length is about ten times that of steel . Similar to carbon fibers, the fibers have a negative coefficient of thermal expansion in the direction of the fibers, i.e. they become shorter and thicker when heated. Their specific strength and their modulus of elasticity are significantly lower than those of carbon fibers. In connection with the positive expansion coefficient of the matrix resin, highly dimensionally stable components can be manufactured. Compared to carbon fiber reinforced plastics, the compressive strength of aramid fiber composites is significantly lower; the impact strength is much higher.
Aramids are extremely heat-resistant, they can easily withstand temperatures of over 370 ° C without melting.
When handling and processing, the slight moisture absorption and the low UV resistance must be taken into account. The fibers lose their strength when exposed to UV radiation (sunlight). The fibers can absorb up to 7% water depending on their storage. Fibers with too much moisture can be dried. Special micro-toothed cutting tools are required to cut aramid fibers. The mechanical processing of finished fiber composite components is also carried out with high-quality processing tools or by water jet cutting . Fiber composite parts are usually made with epoxy resins .
use
- Poly ( p- phenylene terephthalamide): para-aramid fibers are used in fiber-plastic composites . In the security area, they serve as splinter protection, bullet-proof vests , protective helmets , armor for vehicles and cut protection gloves. They are also used in fiber-reinforced plastics in aircraft construction, especially for the construction of gliders . Almost all modern jet engines feature aramid fabrics in the engine cowling. Aramid fibers are used as a substitute for asbestos in brake and clutch linings and seals, as well as reinforcing materials for fiber optic cables or rubber materials , for example . Aramid fabrics are also used in construction, including for stadium roofs.
- Aramid fibers are also often used in sports equipment because of their toughness and tensile strength and their low mass, for example for accessory cords , suspension lines for paragliders , for sails of sailboats .
- The carcass of certain bicycle tires is protected from the penetration of broken glass and the like with aramid inserts. Instead of two steel wire or rope rings, folding tires contain rings made from a winding made of Kevlar yarn or fibers.
- Due to the insufficient elasticity in the event of a fall, "dynamic" climbing ropes cannot be made from aramid.
- Poly ( m -phenylene isophthalamide): Meta-aramid fibers are used specifically for fire protection. They have become known in fire-proof clothing (such as protective suits for fire brigades , racing driver suits , etc.). Another application for aramid is the processing in fiber composite materials into sandwich honeycomb cores . Another field of application for aramid papers (laminates with paper) is electrical insulation. The products are used as cover slides , slot insulation and phase insulation in electric motors and as layer insulation in transformers.
literature
- Philip G. Rose and Karlheinz Hillermeier: Carbon and aramid fiber reinforced plastics. VDI-Gesellschaft Kunststoffe, VDI-Verlag 1977, ISBN 978-3-18-404027-7 .
- Blumberg, Hillermeier, Krüger: Aramid Process. In: Melliand textile reports. 1982.
- Karlheinz Hillermeier, Ulrich Stöcker, Werner Damerau, Joachim Granal: Substitution of asbestos by aramid fibers ... Technical information center Karlsruhe, Federal Ministry for Research and Technology. ISSN 0340-7608 .
- H. Rohrens, K. Hillermeier: Aramid Fibers as Substitute for Asbestos in Seals, Packings and Compensators. In: Magazine: Rubber Fibers Plastics. (GAK) 1984.
Individual evidence
- ↑ Menachem Lewin (Ed.): Handbook of Fiber Chemistry. Third edition. Taylor & Francis Group, Boca Raton 2007, ISBN 978-0-8247-2565-5 , pp. 977f.
- ↑ Commercial Practices, Part 303, §303.7 Generic names and definitions for manufactured fibers.
- ↑ European Textile Labeling Regulation , Regulation (EU) No. 1007/2011 (PDF) Appendix I
- ↑ Walter Loy: Chemical fibers for technical textile products. 2nd, fundamental revised and expanded edition. Deutscher Fachverlag, Frankfurt am Main 2008, ISBN 978-3-86641-197-5 , pp. 107/108.
- ↑ SL Kwolek, HH Yang: History of Aramid Fibers. In: B. Seymour, Rogers S. Porters (Eds.): Manmade Fibers: Their Origin and Development. Elsevier Applied Science, London and New York 1993, ISBN 1-85166-888-8 p. 316.
- ↑ Walter Loy: Chemical fibers for technical textile products. 2nd, fundamental revised and expanded edition. Deutscher Fachverlag, Frankfurt am Main 2008, ISBN 978-3-86641-197-5 , p. 77.
- ↑ Stefan Mecheels, Herbert Vogler, Josef Kurz: Culture and industrial history of textiles . Wachter GmbH, Bönnigheim 2009, ISBN 978-3-9812485-3-1 , p. 445.
- ↑ Hermann Klare: History of chemical fiber research. Akademie-Verlag, Berlin 1985, p. 311.
- ↑ Hermann Klare: History of chemical fiber research. Akademie-Verlag, Berlin 1985, p. 310/311.
- ^ Sanford L. Moskowitz: Advanced Materials Innovation - Managing Global Technology in the 21st century. John Wiley & Sons, Hoboken 2016, ISBN 9780470508923 , p. 75.
- ↑ LeRoy K. McCune: HT-1 High-Temperature-Resistant Polyamide Fibers and Paper. Paper at Thirty-Second Annual Meeting of Textile Research Institute, New York City, March 21, 1962.
- ↑ Paul Winthrop Morgan. Synthetic Polymer Fibrid Paper. US Patent 2999788 [1]
- ↑ Karel F. Mulder: The Other Aramid Fibers. In: B. Seymour, Rogers S. Porters (Eds.): Manmade Fibers: Their Origin and Development. Elsevier Applied Science, London and New York 1993, ISBN 1-85166-888-8 , pp. 337 f.
- ^ Herbert Blades: DRY-JET WET SPINNING PROCESS. U.S. Patent 3,767,756. [ Https://patents.google.com/patent/US3767756A/en ]
- ↑ SL Kwolek, HH Yang: History of Aramid Fibers. In: B. Seymour, Rogers S. Porters (Eds.): Manmade Fibers: Their Origin and Development. Elsevier Applied Science, London and New York 1993, ISBN 1-85166-888-8 p. 317.
- ↑ Karel F. Mulder: The Other Aramid Fibers. In: B. Seymour, Rogers S. Porters (Eds.): Manmade Fibers: Their Origin and Development. Elsevier Applied Science, London and New York 1993, ISBN 1-85166-888-8 , p. 346.
- ↑ SL Kwolek, HH Yang: History of Aramid Fibers. In: B. Seymour, Rogers S. Porters (Eds.): Manmade Fibers: Their Origin and Development. Elsevier Applied Science, London and New York 1993, ISBN 1-85166-888-8 p. 336.
- ↑ Anthony R. Bunsell (Ed.): Handbook Terlon of Properties of Textile and Technical Fibers. 2nd Edition. Elsevier Ltd. 2018, ISBN 978-0-08-101272-7 , p. 626.
- ↑ Karel F. Mulder: The Other Aramid Fibers. In: B. Seymour, Rogers S. Porters (Eds.): Manmade Fibers: Their Origin and Development. Elsevier Applied Science, London and New York 1993, ISBN 1-85166-888-8 , pp. 351/352.
- ↑ Karel F. Mulder: The Other Aramid Fibers. In: B. Seymour, Rogers S. Porters (Eds.): Manmade Fibers: Their Origin and Development. Elsevier Applied Science, London and New York 1993, ISBN 1-85166-888-8 , pp. 353-356.
- ↑ SL Kwolek, HH Yang: History of Aramid Fibers. In: B. Seymour, Rogers S. Porters (Eds.): Manmade Fibers: Their Origin and Development. Elsevier Applied Science, London and New York 1993, ISBN 1-85166-888-8 p. 336.
- ↑ Tejin aramid [2]
- ↑ Kolon Industries - p-Aramid Heracron [3]
- ↑ Tayho Advanced Materials [4]
- ↑ Bernd Tieke: Macromolecular Chemistry. 3rd edition, Wiley-VCH, Weinheim, 2014, ISBN 978-3-527-66227-2 , p. 31 ff.
- ↑ heise online: Google: The internet is being attacked by sharks. Retrieved February 5, 2019 .
- ↑ Nomex types


