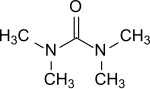
Tetramethylurea
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Tetramethylurea | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 5 H 12 N 2 O | |||||||||||||||
| Brief description |
clear colorless liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 116.16 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density | ||||||||||||||||
| Melting point |
−1 ° C |
|||||||||||||||
| boiling point |
|
|||||||||||||||
| Vapor pressure |
13.3 hPa at 61 ° C |
|||||||||||||||
| solubility |
completely miscible with water (1000 g · l −1 at 20 ° C ) and in most organic solvents |
|||||||||||||||
| Refractive index |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Tetramethylurea ( TMU , from English tetramethylurea ) is a solvent for organic compounds, especially for aromatics , and is suitable as an aprotic- dipolar reaction medium, e.g. B. for Grignard compounds .
The acute toxicity of TMU is moderate. However, it has been shown to be embryotoxic and teratogenic in several animal species and should therefore be used with appropriate safety precautions.
Occurrence and representation
The synthesis and properties of tetramethylurea were extensively described by Arthur Lüttringhaus in 1963.
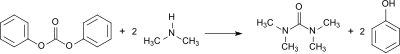
The synthesis variant preferred by the authors is based on the diphenyl carbonate used for the production of polycarbonates , which is reacted with gaseous dimethylamine in the autoclave and gives a tetramethylurea yield of 74%.
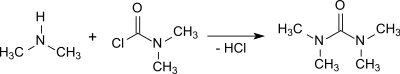
In the reaction of dimethylcarbamoyl chloride with anhydrous sodium carbonate , TMU is obtained in a yield of 96.5%. This reaction was described by Wilhelm Michler and co-workers as early as 1879 .
Dimethylcarbamoyl chloride also reacts with excess dimethylamine to form (contaminated and malodorous) tetramethylurea, which can be purified by adding calcium oxide and subsequent fractional distillation.
The reaction of dimethylamine with phosgene in the presence of z. B. 50% sodium hydroxide solution and subsequent extraction with 1,2-dichloroethane gives TMU in 95% yield.
The reactions with dimethylcarbamoyl chloride and phosgene are strongly exothermic and the removal of the dimethylamine hydrochloride produced requires some effort.
Tetramethylurea is also formed during the oxidation of tetrakis (dimethylamino) ethylene (TDAE), a very electron-rich alkene and strong reducing agent, accessible from tris (dimethylamino) methane by pyrolysis or from chlorotrifluoroethene and dimethylamine.
TDAE reacts with oxygen in a (2 + 2) cycloaddition to form a 1,2-dioxetane , which breaks down into electronically excited tetramethylurea. This returns to the ground state, emitting green light with an emission maximum at 515 nm.
properties
Tetramethylurea is a clear, colorless liquid with a faint aromatic odor that mixes with water and many organic solvents. The liquid state of TMU and its wide liquid range of> 170 ° C is unusual for ureas.
Applications
Tetramethylurea is miscible with a large number of organic compounds, including acids such as. B. acetic acid or bases, such as. B. pyridine and an excellent solvent for organic substances such. B. ε-caprolactam or benzoic acid and itself dissolves some inorganic salts, such as. B. silver nitrate or sodium iodide . Due to its pronounced solvent properties, TMU is often used as a replacement for the carcinogenic hexamethylphosphoric acid triamide (HMPT).
Tetramethylurea is suitable as a reaction medium for the polymerization of aromatic diacid chlorides, such as. B. isophthalic acid dichloride and aromatic diamines , such as. B. 1,3-diaminobenzene (m-phenylenediamine) to aramids such. B. poly (m-phenylene isophthalamide) (Nomex®).
The polymerization of 4-aminobenzoic acid chloride hydrochloride in TMU provides isotropic, viscous solutions of poly (p-benzamide) (PPB), which can be spun directly into fibers.
Stable isotropic solutions up to a PPB polymer concentration of 14% can be obtained in a TMU- LiCl mixture .
TMU also dissolves cellulose esters and swells other polymers such as. B. polycarbonates, polyvinyl chloride or aliphatic polyamides , usually at elevated temperature.
Strong hindered and non-nucleophilic guanidine bases are easily accessible from tetramethylurea ,
which, in contrast to the fused amidine bases DBN or DBU, are not alkylated.
A modification of the Koenigs-Knorr method for the construction of glycosides from 2,3,4,6-tetra- O -acetyl-α- D -glucopyranosyl bromide (acetobromoglucose) comes from S. Hanessian, who used silver trifluoromethanesulfonate (Ag triflate TfOAg) and tetramethylurea as a proton acceptor. This process variant is characterized by a simplified process management, as well as high anomer purity and yields of the products.
If the reaction is carried out with acetobromoglucose and silver triflate / TMU at room temperature, then TMU reacts not only as a base, but also with the glycosyl halide to form an easily isolable uronium triflate in 56% yield.
Individual evidence
- ↑ a b c d data sheet tetramethylurea from Sigma-Aldrich , accessed on August 15, 2016 ( PDF ).
- ↑ a b c d e f g Entry on tetramethylurea in the GESTIS substance database of the IFA , accessed on January 10, 2017(JavaScript required) .
- ↑ a b Tetramethylurea data sheet at AlfaAesar, accessed on August 15, 2016 ( PDF )(JavaScript required) .
- ↑ a b Patent US3681457 : Method of making tetramethylurea. Applied on February 26, 1969 , published August 1, 1972 , applicant: The Ott Chemical Co., inventor: H. Babad.
- ↑ a b R.M. Giuliano: tetramethylurea . In: e-EROS Encyclopedia of Reagents for Organic Synthesis . 2004, doi : 10.1002 / 047084289X.rn00399 .
- ↑ a b c d e A. Lüttringhaus, H.-W. Dirksen: tetramethylurea as solvent and reaction partner . In: Angew. Chem. Band 75 , no. 22 , 1963, pp. 1059-1068 , doi : 10.1002 / anie.19630752204 .
- ↑ Tetramethylurea data sheet (PDF) from Merck , accessed on August 15, 2016.
- ^ The MAK Collection for Occupational Health and Safety: Tetramethylurea [MAK Value Documentation in German language, 1979], Documentations and Methods . Wiley-VCH, Weinheim 2012, doi : 10.1002 / 3527600418.mb63222d0007 .
- ^ JK Lawson, Jr., JAT Croom: Dimethylamides from alkali carboxylates and dimethylcarbamoyl chloride . In: J. Org. Chem. Band 28 , no. 1 , 1963, p. 232-235 , doi : 10.1021 / jo1036a513 .
- ↑ W. Michler, C. Escherich: Ueber multiply substituted ureas . In: Ber. German Chem. Ges. Volume 12 , no. 1 , 1879, p. 1162–1164 , doi : 10.1002 / cber.187901201303 .
- ↑ Patent US3597478 : Preparation of tetramethylurea. Filed September 14, 1967 , published August 3, 1971 , applicant: Nipak, Inc., inventor: ML Weakly.
- ↑ H. Bock, H. Borrmann, Z. Havlas, H. Oberhammer, K. Ruppert, A. Simon: Tetrakis (dimethylamino) ethene: An extremely electron-rich molecule with an unusual structure both in the solid state and in the gas phase . In: Angew. Chem. Band 103 , no. 12 , 1991, pp. 1733-1735 , doi : 10.1002 / anie.19911031246 .
- ↑ H. Weingarten, WA White: Synthesis of Tetrakis (dimethylamino) ethylene . In: J. Org. Chem. Band 31 , no. 10 , 1966, pp. 3427-3428 , doi : 10.1021 / jo01348a520 .
- ↑ Patent US3293299 : Process for making tetrakis (dimethylamino) ethylene. Applied on October 4, 1965 , published December 20, 1966 , applicant: EI du Pont de Nemours and Co., inventor: H. Boden.
- ^ HE Winberg, JR Downing, DD Coffman: The chemiluminescence of tetrakis (dimethylamino) ethylene . In: J. Am. Chem. Soc. tape 87 , no. 9 , 1965, pp. 2054-2055 , doi : 10.1021 / ja01087a039 .
- ↑ Chemiluminescence from TDAE. illumina-chemie.de, August 8, 2014, accessed on August 22, 2016 .
- ^ BJ Barker, JA Caruso: The Chemistry of Nonaqueous Solvents, IV. Solution Phenomena and Aprotic Solvents . Academic Press, New York 1976, ISBN 0-12-433804-6 , pp. 110-127 .
- ↑ BJ Barker, J. Rosenfarb, JA Caruso: Urea as a solvent in chemical research . In: Angew. Chem. Band 91 , no. 7 , 1979, pp. 560-564 , doi : 10.1002 / anie.19790910707 .
- ↑ AJ Chalk: The use of sodium hydride as a reducing agent in nitrogen-containing solvents I. The reduction of chlorosilanes in Hexaalkylphosphoric triamides and tetraalkylureas . In: J. Organomet. Chem. Band 21 , no. 1 , 1970, p. 95-101 , doi : 10.1016 / S0022-328X (00) 90598-9 .
- ↑ G. Odian: Principles of Polymerization, 4th Edition . Wiley-Interscience, Hoboken, NJ 2004, ISBN 0-471-27400-3 , pp. 100 .
- ↑ HG Rodgers, RA Gaudiana, WC Hollinsed, PS Kalyanaraman, JS Manello, C. McGovern, RA Minns, R. Sahatjian: Highly amorphous, birefringent, para-linked aromatic polyamides . In: Macromolecules . tape 18 , no. 6 , 1985, pp. 1058-1068 , doi : 10.1021 / ma00148a003 .
- ^ J. Preston: Synthesis and Properties of Rodlike Condensation Polymers, in Liquid Crystalline Order in Polymers . Ed .: A. Blumstein. Academic Press, New York 1978, ISBN 0-12-108650-X , pp. 141-166 .
- ↑ SL Kwolek, PW Morgan, JR Schaefgen, LW Gulrich: Synthesis, Anisotropic Solutions, and Fibers of Poly (1,4-benzamide) . In: Macromolecules . tape 10 , no. 6 , 1977, pp. 1390-1396 , doi : 10.1021 / ma60060a041 .
- ↑ DHR Barton, M. Chen, JC Jászbérenyi, DK Taylor: PREPARATION AND REACTIONS OF 2-tert-BUTYL-1,1,3,3-TETRAMETHYLGUANIDINE: 2,2,6-TRIMETHYLCYCLOHEXEN-1-YL IODIDE In: Organic Syntheses . 74, 1997, p. 101, doi : 10.15227 / orgsyn.074.0101 ; Coll. Vol. 9, 1998, p. 147 ( PDF ).
- ↑ DHR Barton, JD Elliott, SD Géro: The synthesis and properties of a series of strong but hindered organic bases . In: J. Chem. Soc., Chem. Commun. 1981, p. 1136-1137 , doi : 10.1039 / C39810001136 .
- ↑ S. Hanessian, J. Banoub: Chemistry of the glycosidic linkage. An efficient synthesis of 1,2-trans-disaccharides . In: Carbohydr. Res. Band 53 , 1977, pp. C13-C16 , doi : 10.1016 / S0008-6215 (00) 85468-3 .
- ↑ K. Bock, J. Fernández-Bolanos Guzmán, S. Refn: Synthesis and properties of 1,1,3,3-tetramethyl-2- (2,3,4,6-tetra- O- acetyl-α-D -glucopyranosyl) uronium triflate . In: Carbohydr. Res. Band 232 , 1992, pp. 353-357 , doi : 10.1016 / 0008-6215 (92) 80067-B .