Phenylenediamines
| Phenylenediamines | ||||||||
| Surname | o -phenylenediamine | m -phenylenediamine | p -phenylenediamine | |||||
| other names | 1,2-diaminobenzene |
1,3-diaminobenzene |
1,4-diaminobenzene, CI 76060 |
|||||
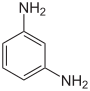
| Structural formula |

|
|
|
|||||
| CAS number | 95-54-5 | 108-45-2 | 106-50-3 | |||||
| ECHA InfoCard | 100.002.210 | 100.003.259 | 100.003.096 | |||||
| PubChem | 7243 | 7935 | 7814 | |||||
| Molecular formula | C 6 H 8 N 2 | |||||||
| Molar mass | 108.14 g mol −1 | |||||||
| Physical state | firmly | |||||||
| Brief description | colorless to pale red crystals | |||||||
| Melting point | 102.1 ° C | 63 ° C | 140 ° C | |||||
| boiling point | 257 ° C | 284 ° C | 267 ° C | |||||
|
pK s 1 value (of the conjugate acid BH + ) |
4.74 | 4.98 | 6.2 | |||||
|
pK s 2 value (of the conjugate acid BH + ) |
0.6 | 2.41 | 2.67 | |||||
| solubility | 54 g l −1 (20 ° C) | 429 g l −1 (20 ° C) | 40 g l −1 (24 ° C) | |||||
| moderately to well soluble in water, well in organic solvents | ||||||||
|
GHS labeling |
|
|
||||||
| H and P phrases |
301-312 + 332-317 319-341-351-410 |
301 + 311 + 331-317 319-341-410 |
301-311-331 319-317-410 |
|||||
| no EUH phrases | no EUH phrases | no EUH phrases | ||||||
|
273-280-302 + 352 305 + 351 + 338-308 + 310 |
261-273-280-301 + 310 305 + 351 + 338-311 |
280-273-304 + 340-302 + 352 305 + 351 + 338-309 + 310 |
||||||
| MAK | Switzerland: 0.1 mg m −3 (measured as inhalable dust ) | |||||||
| toxicity | 720–1600 mg kg −1 ( LD 50 , rat , oral ) |
280 mg kg −1 ( LD 50 , rat , oral ) |
80 mg kg −1 ( LD 50 , rabbit , transdermal ) |
|||||
The phenylenediamines (also diaminobenzenes ; abbreviation PPD or PDA) are chemical compounds from the group of aromatic amines and important starting materials for many organic compounds . They consist of a benzene ring with two amino groups (–NH 2 ). By different arrangements of these groups, there are three structural isomers : 1,2-phenylenediamine ( o rtho-phenylenediamine), 1,3-phenylenediamine ( m eta-phenylenediamine), and 1,4-phenylenediamine ( p ara-phenylenediamine).
Extraction and presentation
The phenylenediamines are prepared by reducing the nitroanilines with hydrogen in toluene as a solvent in the presence of a catalyst and purified by distillation . Reduction of m-dinitrobenzene with Fe / HCl leads to m- phenylenediamine.
properties
The phenylenediamines form colorless to pale red crystals, which oxidize quickly in the air and turn brown in the process ( o- phenylenediamine: formation of o-quinonediimine and further 2,3-diaminophenazine ). The stable dihydrochlorides of the compounds are therefore often used. o -Phenylenediamine condenses with ketones and aldehydes to form Schiff's bases . This reaction allows the synthesis of substituted benzimidazoles . Quinoxalinedione can be produced by condensation of o -phenylenediamine with dimethyl oxalate .
The phenylenediamines are crystalline solids. Their melting points differ significantly. p- phenylenediamine, which has the highest symmetry, has the highest melting point.
Water is not very suitable as a solvent . In contrast, they dissolve well in many organic solvents.
use
o -Phenylenediamine is used as a starting material or intermediate for the synthesis of heterocyclic compounds, especially benzotriazole and phenazine . Furthermore, o- phenylenediamine is used in the production
- of benzotriazoles, which for the production of plastics and photographic chemicals, as well as corrosion inhibitors for copper are used
- of pesticides (e.g. as benzimidazole, phenylenediamine thiourea carbamate and quinoxaline ), dyes and pigments
- of benzimidazole derivatives for UV absorbers in cosmetics, thermostable polymers and pharmaceuticals (for virucides and anthelmintics used in veterinary use)
- of 2-mercaptobenzimidazole (rubber antioxidant)
- of hair colors
- as a substrate in the ELISA reagent in biotechnological laboratories
Certain derived compounds of o- phenylenediamine may be used in cosmetics and are probably the cause of many allergic symptoms in hairdressers.
The plastics industry produces large quantities of p- phenylenediamine for the manufacture of aromatic polyamides . Together with terephthalic acid, it is an essential component of the aramids . p- phenylenediamine is an intermediate product in the manufacture of azo dyes . The synthetic pathways for some pharmaceuticals and photochemicals are just as often via this compound. Because of its coloring properties, p- phenylenediamine and some of its derivatives are used in cosmetics (especially for hair dyes, this use was discovered by Ernst Erdmann in 1888 ). Presumably, many hairdresser allergic reactions are based on contact with this dye . In some photographic developers , the alkaline solution of p -phenylenediamine is the active component.
safety instructions
o -Phenylenediamine is classified as a carcinogen according to category 3B and as a skin sensitizer. The vapor of o- phenylenediamine forms an explosive mixture with air. p -phenylenediamine is counted as an allergen because it can cause irritation and sensitization if it comes into contact with the skin . In addition, this chemical is also used in some holiday resorts to intensify the color and to “blacken” red-brown henna tattoos .
m -Phenylenediamine leads to the formation of methemoglobin and thus to the breakdown of red blood cells, which can result in liver and kidney damage. The substance can be absorbed as dust via the respiratory tract, eyes and skin.
Individual evidence
- ↑ a b c d Entry for CAS no. 95-54-5 in the GESTIS substance database of the IFA , accessed on December 27, 2019(JavaScript required) .
- ↑ a b c d e Entry for CAS no. 108-45-2 in the GESTIS substance database of the IFA , accessed on December 27, 2019(JavaScript required) .
- ↑ a b c d e Entry for CAS no. 106-50-3 in the GESTIS substance database of the IFA , accessed on December 27, 2019(JavaScript required) .
- ↑ a b CRC Handbook of Tables for Organic Compound Identification , Third Edition, 1984, ISBN 0-8493-0303-6 .
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for phenylenediamine ), accessed on November 25, 2019.
- ↑ Data sheet from the Federal Agency for Environmental Protection.
- ^ Hans Beyer and Wolfgang Walter : Textbook of Organic Chemistry , 19th edition, S. Hirzel Verlag, Stuttgart 1981, ISBN 3-7776-0356-2 , pp. 536, 542.
- ↑ data sheet from Enius .
- ↑ Entry on phenylenediamines. In: Römpp Online . Georg Thieme Verlag, accessed on April 12, 2015.



