Dimethyl oxalate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
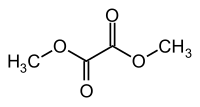
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Dimethyl oxalate | |||||||||||||||
| other names |
Dimethyl oxalate |
|||||||||||||||
| Molecular formula | C 4 H 6 O 4 | |||||||||||||||
| Brief description |
colorless solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 118.09 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.148 g cm −3 (25 ° C) |
|||||||||||||||
| Melting point |
50-54 ° C |
|||||||||||||||
| boiling point |
163.5 ° C |
|||||||||||||||
| solubility |
|
|||||||||||||||
| Refractive index |
1.39 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Thermodynamic properties | ||||||||||||||||
| ΔH f 0 |
-756.3 kJ / mol |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Dimethyl oxalate is a chemical compound from the group of oxalates , an ester of oxalic acid .
Extraction and presentation
Dimethyl oxalate can be obtained by esterifying oxalic acid with methanol using p-toluenesulfonic acid as a catalyst .
It can also be prepared by oxidative carbonylation .
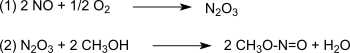
In a Pd 2+ -catalyzed reaction under relatively mild process conditions and with high yields, the oxidative carbonylation of methanol combines classic C 1 building blocks from the synthesis gas obtained from coal or biomass to form the C 2 building block dimethyl oxalate. The oxidation takes place via nitrous oxide , which is formed from nitrogen monoxide and oxygen according to (1) and then reacts with methanol to form methyl nitrite according to (2) :
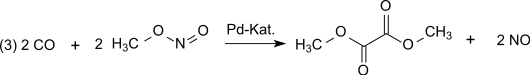
In the next reaction step (3) of the dicarbonylation, carbon monoxide reacts with methyl nitrite in the vapor phase at normal pressure and temperatures of 80–120 ° C on a palladium contact to form dimethyl oxalate:
The sum equation shows that oxygen acts as the actual oxidizing agent via the reactants dinitrogen trioxide and methyl nitrite.
With regard to methyl nitrite, which functions as a carrier of the oxidation equivalents, the process runs without losses. However, the resulting water must be drained off, otherwise hydrolysis of the dimethyl oxalate is to be expected. Interestingly, the course of the reaction depends on X.-Z. Jiang depends crucially on the nature of the carrier material on which the palladium catalyst is applied. With 1% Pd / α-Al 2 O 3 , dimethyl oxalate is selectively formed in a dicarbonylation reaction, with 2% Pd / C under the same reaction conditions, dimethyl carbonate is formed by monocarbonylation according to:
Alternatively, the oxidative carbonylation of methanol can also be carried out with 1,4-benzoquinone (BQ) as the oxidizing agent in the Pd (OAc) 2 / PPh 3 / BQ system with a mass ratio of 1/3/100 at 65 ° C. and 70 atm. CO with high yield and selectivity can be carried out:
properties
Dimethyl oxalate is a colorless solid that is soluble in water.
use
Dimethyl oxalate is used for alkylation and as a chelating agent in the cosmetics industry.
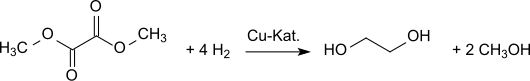
For countries with low oil reserves but large coal reserves (in the future perhaps also biomass availability), i. H. Countries with great potential for synthesis gas-based chemistry, such as B. China, the oxidative carbonylation of methanol offers a new and promising access to the important C 2 base chemical ethylene glycol Dimethyl oxalate can be converted into ethylene glycol in very good yields (94.7% of theory) by hydrogenation on copper-containing catalysts:
The resulting methanol is returned to the oxidative carbonylation process; d. In other words, the only raw materials used in the overall process are carbon monoxide, hydrogen and oxygen. A plant with a capacity of 200,000 tonnes of ethylene glycol per year based on this “Coal-to-MEG” process is already in operation in Inner Mongolia, and a second plant with 250,000 tonnes per year is scheduled for the second half of 2012 in Henan Province in Go into operation. Further plants with a total annual capacity of more than 1 million tons of ethylene glycol are planned.
Dimethyl carbonate, discussed as a fuel additive (so-called oxygenate) from biomass, can also be obtained from dimethyl oxalate by decarbonylation at temperatures around 100 ° C in the presence of alkali metal alcoholates according to:
The resulting carbon monoxide can be returned to the initial reaction (3).
By transesterification of dimethyl oxalate with phenol in the presence of titanium catalysts diphenyl oxalate obtained, analogous to the dimethyl oxalate in the liquid or gas phase to diphenyl carbonate is decarbonylated. Diphenyl carbonate is used to replace the highly toxic phosgene in the manufacture of polycarbonates .
Related links
- Diethyl oxalate C 6 H 10 O 4
Individual evidence
- ↑ a b c d e f g data sheet Dimethyl oxalate from Sigma-Aldrich , accessed on October 16, 2016 ( PDF ).
- ↑ a b Entry on dimethyl oxalate at ChemBlink , accessed on February 25, 2011.
- ↑ Entry on Dimethyl oxalate at TCI Europe, accessed on June 27, 2011.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Standard Thermodynamic Properties of Chemical Substances, pp. 5-26.
- ^ Hans-Jürgen Arpe: Industrial organic chemistry: Significant preliminary and intermediate products , p. 168; ISBN 978-3-527-31540-6 .
- ↑ Patent US4467109 : Process for continuous preparation of diester of oxalic acid. Filed on May 19, 1983 , published on August 21, 1984 , Applicant: Ube Industries , inventor Susumu Tahara et al .. and EP108359, Process for the preparation of a diester of oxalic acid , inventors K. Masunaga et al. , Applicant: Ube Industries, Ltd., published May 16, 1984
- ↑ European patent application EP-A 425 197, Process for preparing diester of carbonic acid , inventors: K. Nishihira, K. Mizutare, applicant: Ube Industries, published on May 2, 1991
- ↑ U.S. Patent 4,451,666, Synthesis of oxalate esters by the oxidative carbonylation of alcohols , inventor: JA Sofranko, AM Gaffney, assignee: Atlantic Richfield Co., issued May 29, 1984.
- ↑ a b E. Amadio: Oxidative Carbonylation of Alkanols Catalyzed by Pd (II) -Phosphine Complexes , PhD Thesis, Ca'Foscari University Venice, 2009.
- ↑ a b X.-Z. Jiang, Palladium Supported Catalysts in CO + RONO Reactions , Platinum Metals Rev., 1990, 34 , (4), 178-180
- ↑ alkylation with Oxalic acid ester. Scope and Mechanism
- ↑ Marina Bährle-Rapp: Springer Lexicon Cosmetics and Body Care , p. 130; ISBN 978-3-540-20416-9 .
- ↑ Nexant / Chemsystems, Coal to MEG, Changing the Rules of the Game ( Memento of July 14, 2011 in the Internet Archive ) (PDF; 5.4 MB), 2011 Prospectus
- ↑ European patent application EP-A 046 983, Process for continuously preparing ethylene glycol , inventor: S. Tahara et al., Applicant: Ube Industries, Ltd., published on March 10, 1982, and HT Teunissen and CJ Elsevier, Ruthenium catalyzed hydrogenation of dimethyl oxalate to ethylene glycol , J. Chem. Soc., Chem. Commun., 1997, 667-668).
- ↑ S. Zhang et al., Highly-Dispersed Copper-Based Catalysts from Cu – Zn – Al Layered Double Hydroxide Precursor for Gas-Phase Hydrogenation of Dimethyl Oxalate to Ethylene Glycol , Catalysis Letters, Sept. 2012, 142 (9), 1121 -1127.
- ^ C. Boswell, China's coal-based chemicals are a trade-off , ICIS Chemical Business , dated January 20, 2010.
- ↑ Fund of the Chemical Industry - Information Series Renewable Raw Materials: Family Tree Biomass Products (PDF; 99 kB)
- ↑ U.S. Patent 4,544,507, Production of carbonate diesters from oxalate diesters , inventor: P. Foley, assignee: Celanese Corp., issued October 1, 1985
- ↑ U.S. Patent 5,834,615, Process for producing diaryl carbonate , inventor: K. Nishihira et al., Applicant: Ube Industries, Ltd., issued November 10, 1998, and XB Ma et al., Preparation of Diphenyl Oxalate from Transesterification of Dimethyl Oxalate with Phenol over TS-1 Catalyst , Chinese Chem. Lett., 14 (5), 461-464 (2003).
- ↑ Japanese Patent Application JP-2011236146, Method for Producing Diaryl Carbonate and Method for Producing Polycarbonate , Applicant: Mitsubishi Chemical Corp., published November 24, 2011.