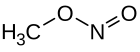
Methyl nitrite
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Methyl nitrite | |||||||||||||||
| other names |
Methyl nitrous acid |
|||||||||||||||
| Molecular formula | CH 3 NO 2 | |||||||||||||||
| Brief description |
colorless gas |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 61.04 g · mol -1 | |||||||||||||||
| Physical state |
gaseous |
|||||||||||||||
| density |
0.991 g cm −3 |
|||||||||||||||
| Melting point |
−40.2 ° C |
|||||||||||||||
| boiling point |
−6.42 ° C |
|||||||||||||||
| solubility |
very poorly soluble |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Thermodynamic properties | ||||||||||||||||
| ΔH f 0 |
−66.1 kJ / mol |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Methyl nitrite , is an ester of nitrous acid with methanol and is a colorless, extremely flammable and poisonous gas at room temperature. It is the most simply structured alkyl nitrite.
presentation
Methyl nitrite is formed when cold, dilute sulfuric acid acts on a water- methanol solution of sodium nitrite . Methyl nitrite (mixed with nitromethane) can also be produced by reacting silver nitrite with iodomethane .
Another manufacturing option is the addition of a mixture of nitrosylsulfuric acid and sulfuric acid in aqueous methanol.
properties
Methyl nitrite has a melting point of −40.2 ° C and a boiling point of −6.42 ° C. In the older literature these are given as −16 ° C (m.p.) and −12 ° C (b.p.). According to August, the vapor pressure function results according to lg (P) = −A / T + B (P in Torr, T in K) with A = 1365 and B = 8.102 in the temperature range from 154 K to 225 K. From the vapor pressure function a Derive the molar enthalpy of vaporization of 26.15 kJ mol −1 . As is usual with alkyl nitrites, the action of nascent hydrogen on the compound produces methanol, ammonia and water.
structure
At room temperature, methyl nitrite consists of a mixture of the cis - and the trans - conformer . The cis conformer is 3.13 kJ mol −1, more stable than the trans form. The energy barrier for the internal rotation is 45.3 kJ · mol −1 .
Explosion limits
- Lower explosion limit: 5.3 vol%, 137 g / m 3
- Upper explosion limit: 100% by volume, upper explosion limit does not exist as the substance decomposes.
use
For nitrosation in organic synthesis z. B. for the representation of isonitrosopropiophenone.
Individual evidence
- ↑ a b c d e f g h i Entry for CAS no. 624-91-9 in the GESTIS substance database of the IFA , accessed on June 19, 2013(JavaScript required) .
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Standard Thermodynamic Properties of Chemical Substances, pp. 5-20.
- ↑ a b L. F. Fieser, M. Fieser: Organic chemistry. 2nd edition, Verlag Chemie, 1975.
- ↑ Tarte, P .: Rotational Isomerism as a General Property of Alkyl Nitrites in J. Chem. Phys., 1952, 20, pp. 1570-1575. doi : 10.1063 / 1.1700218 .
- ^ Donald L. Pavia, Gary M. Lampman, George S. Kriz: Organic Chemistry , Volume 2. Thompson Custom Publishing, Mason, Ohio 2004, ISBN 0-03-014813-8 .
- ↑ a b c Rook, FL: Preparation, Vapor Pressure, and Infrared Spectrum of Methyl Nitrite in J. Chem. Eng. Data 27 (1982) pp. 72-73, doi : 10.1021 / je00027a022 .
- ^ Beyer / Walter: Textbook of Organic Chemistry , 22nd edition, S. Hirzel Verlag, Stuttgart 1991, p. 154.
- ↑ BJ Van der Veken, R. Maas, GA Guirgis, HD Stidham, TG Sheehan, JR Durig: Infrared spectrum, ab initio calculations, barriers to internal rotation and structural parameters for methyl nitrite . In: J. Phys. Chem. . 94, No. 10, 1990, pp. 4029-4039. doi : 10.1021 / j100373a028 .
- ^ Walter H. Hartung, Frank Crossley: Isonitrosopropiophenone In: Organic Syntheses . 16, 1936, p. 44, doi : 10.15227 / orgsyn.016.0044 ; Coll. Vol. 2, 1943, p. 363 ( PDF ).
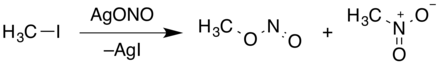
![{\ displaystyle \ mathrm {CH_ {3} ONO \ + \ 6 [H] \ longrightarrow \ CH_ {3} OH \ + \ NH_ {3} \ + \ H_ {2} O}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/9295bdb62b0542ca837e1f639e67c861749819fa)

