2,3,4,6-tetra-O-acetyl-α- D -glucopyranosyl bromide
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | 2,3,4,6- tetra- O -acetyl-α- D -glucopyranosyl bromide | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 14 H 19 BrO 9 | ||||||||||||||||||
| Brief description |
white to pale yellowish crystal powder |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 411.20 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
|
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
2,3,4,6- Tetra- O -acetyl-α- D -glycopyranosyl bromide (acetobromoglucose) is a so-called glycosyl halide and, as a glycosyl donor, a standard reagent for glycosylation reactions in carbohydrate chemistry . Acetobromoglucose reacts with suitable glycosyl acceptors in the presence of silver salts according to the Koenigs-Knorr method , forming glycosidic bonds to form glucosides , a subgroup of glycosides .
Occurrence and representation
In their fundamental publication from 1901, Wilhelm Koenigs and Eduard Knorr described the synthesis of 2,3,4,6- tetra- O- acetyl-α- D -glycopyranosyl bromide from glucose and acetyl bromide in one 58% pure yield.
The use of the relatively expensive and uncomfortable to handle acetyl bromide avoids the two-stage synthesis of Emil Fischer , in glucose first with acetic anhydride and sodium acetate to pentaacetyl glucose (74% yield) and this then with hydrobromic acid in quantitative yield or with a saturated solution of hydrogen bromide in Glacial acetic acid is converted to acetobromoglucose in 76% yield.
In the reaction of glucose with acetic anhydride which has been saturated with gaseous hydrogen bromide, pure yields of acetobromoglucose of 50 to 60% are also achieved.
The standard procedure developed from the early syntheses of acetobromoglucose also runs via pentaacetylglucose, which reacts with gaseous hydrogen bromide in a crude yield of 80 to 87% to form the end product.
The use of gaseous hydrogen bromide avoids a reaction variant in which pentaacetylglucose in chloroform is first mixed with red phosphorus and bromine with intermediate formation of phosphorus tribromide , which then reacts to phosphonic acid and hydrogen bromide by adding water . The resulting acetobromoglucose changes into the chloroform phase and is isolated in a yield of 84% (based on pentaacetylglucose).
properties
2,3,4,6- Tetra- O -acetyl-α- D -glycopyranosyl bromide is a white to pale yellow, odorless crystal powder and when recrystallized from petroleum ether or diisopropyl ether forms "radiant, shiny, white needles" that are in water decompose and are soluble in many organic solvents. Acetobromoglucose is stable for months under exclusion of light in a vacuum desiccator over sodium hydroxide ; the connection is strongly clockwise and reduces Fehling's solution .
Applications
Substitution reactions on acetobromoglucose
The simplest reaction of 2,3,4,6- tetra- O- acetyl-α- D -glycopyranosyl bromide is hydrolysis in the sense of a nucleophilic exchange of the bromine for a hydroxyl group in the presence of silver carbonate to form β- D -glucose-2,3 , 4,6-tetraacetate, which is obtained in pure yields of 75 to 80% when reacted in acetone .
In contrast, the reaction of acetobromoglucose with acrylonitrile in the presence of tributyltin hydride under UV radiation is a radical substitution that leads to 1-deoxy-2,3,4,6-tetra- O- acetyl-1- (2 -cyanoethyl) -α-glucopyranose.
The exchange of the bromine atom in 2,3,4,6- tetra- O -acetyl-α- D -glycopyranosyl bromide for fluorine by means of potassium hydrogen difluoride in acetonitrile gives 2,3,4,6- tetra- O -acetyl-α- D -glycopyranosyl fluoride in 70% yield,
which, like acetobromoglucose, is suitable as - albeit less reactive - glycosyl donor.
2,3,4,6- Tetra- O -acetyl-α- D -glycopyranosyl bromide is easily converted to 2,3,4,6- tetra- O- acetyl-1-thio-β- D via the isothiuronium salt glucopyranoside (peracetylated β-thioglucose) accessible for a Schlüsseledukt auranofin , the chronic treatment of rheumatoid arthritis is used.
After splitting off the acetyl groups with sodium methoxide and acidification, 1-thio-β- D -glucopyranoside is obtained.
Thioglycosides (instead of H, R = alkyl, aryl) are also suitable as glycosyl donors (activation by N-iodosuccinimide / silver triflate ) and are characterized by significantly higher stabilities compared to glycosyl halides such as acetobromoglucose.
Acetobromoglucose as a glycosyl donor
Until the discovery of O- glycosyl-trichloroacetimidates, glycosyl halides of the 2,3,4,6- tetra- O- acetyl-α- D -glycopyranosyl bromide type were the ultimate glycosyl donors.
As early as 1901, W. Koenigs and E. Fischer described the preparation of the simplest β-glucoside 2,3,4,6- tetra- O- acetyl-β-methyl- D -glucopyranoside from acetobromoglucose and methanol with insoluble silver salts, e.g. B. silver carbonate, as activators ( promoters ).
As alternative promoters, Burckhardt Helferich and colleagues used mercury salts, such as. B. Mercury (II) cyanide Hg (CN) 2 or mercury (II) bromide HgBr 2 or Hg (CN) 2 / HgBr 2 mixtures are described, which sometimes give better yields and selectivities, but because of the toxicity of the mercury (II) ions and the hydrogen cyanide produced in the so-called Helferich variant of the Koenigs-Knorr method have become largely obsolete.
Longer-chain alcohols C n H 2n + 1 OH with n = 6–13 can also be converted into the corresponding n-alkyl-β- D - in yields of around 60% (after deacetylation with sodium methoxide in methanol) with acetobromoglucose and lithium carbonate as promoters at room temperature . convert glucopyranosiden.
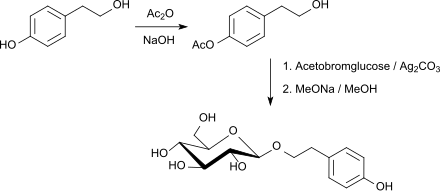
Pharmacologically active natural substances such as B. Salidroside with antidepressant and anti- anxiety properties are obtained by glycosylation of the alcoholic hydroxyl group of tyrosol (4- (2-hydroxyethyl) phenol), an antioxidant from olive oil , with acetobromoglucose and silver carbonate as promoters in a total yield of 72% after splitting off the acetyl groups in kilograms representable.
Acetobromoglucose is also suitable for the glucosylation of phenols - in the much more nucleophilic phenolate form - z. B. from hydroquinone to arbutin , from salicyl alcohol (saligenin) to salicin or from vanillin to 2,3,4,6-tetra- O -acetyl-β- D -glucopyranosylvanillin - in this variant with tetrabutylammonium bromide as a particularly mild promoter in 50% iger yield.
Another modification of the Koenigs-Knorr method for the construction of disaccharides from 2,3,4,6- tetra- O -acetyl-α- D -glucopyranosyl bromide uses the most active promoter silver trifluoromethanesulfonate (Ag triflate, AgOTf) in equimolar amounts and as Proton acceptor tetramethylurea This process variant is characterized by a simplified process control, as well as high anomer purity and yields of the products.
The synthesis of more complex oligosaccharides with the glycosyl donor 2,3,4,6- tetra- O -acetyl-α- D -glycopyranosyl bromide is made possible by its thermal and chemical instability and the use of expensive and toxic heavy metal salts as promoters, of dehydrating agents to bind released water when using the insoluble silver salts silver (I) oxide and silver carbonate, as well as proton acceptors for binding the liberated hydrogen bromide.
While the active Hg and Ag promoters with neighboring group participation of acyl groups on the ring carbon C2 lead preferentially to the kinetically favored β-glycosides (1,2-trans conformation),
finds with weak promoters such as B. Tetraalkylammoniumhalogeniden (R 4 NBr) a so-called " in situ anomerization " takes place with the formation of the (more reactive) β-acetobromoglucose, which leads with glycosyl acceptors to α-linked glycosides (1,2-cis-conformation).
Acetobromoglucose occupies a middle position among the peracetylated glycosyl halides (glycosyl-X) as a glycosyl donor: X: I> Br> Cl> F. The reactivity of glycosyl acceptors decreases from HO-Me >> HO-CH 2 -R> 6- OH >> 3-OH> 2-OH> 4-OH.
Individual evidence
- ↑ a b Entry on 2,3,4,6-Tetra-O-acetyl- D -glycopyranosyl Bromide (stabilized with CaCO 3 ) at TCI Europe, accessed on August 25, 2016.
- ↑ Acetobromo-alpha- D -glucose data sheet from AlfaAesar, accessed on August 25, 2016 ( PDF )(JavaScript required) .
- ↑ a b c d Pfaltz & Bauer: Acetobromo-a- D -glucose , accessed on August 25, 2016.
- ^ A b c d e W. Koenigs, E. Knorr: About some derivatives of dextrose and galactose . In: Chem. Ber. tape 34 , no. 1 , 1901, p. 957-981 , doi : 10.1002 / cber.190103401162 .
- ↑ O. Lockhoff: Glycosyl halides . In: Hoeben-Weyl, Methods of Organic Chemistry . 4th edition. E14a / 3. G. Thieme, Stuttgart 1992, ISBN 978-3-13-776704-6 , p. 626-728 .
- ↑ V. Semeniuchenko, Y. Garazd, M. Garazd, T. Shokol, U. Groth, V. Khilya: Highly efficient glucosylation of flavonoids . In: monthly Chem. Band 140 , no. 12 , 2009, p. 1503-1512 , doi : 10.1007 / s00706-009-0207-6 .
- ^ A. Colley: Some remarks on the treatise of the HHrn. Koenigs and Knorr on derivatives of grape sugar . In: Chem. Ber. tape 34 , no. 2 , 1901, p. 3205-3207 , doi : 10.1002 / cber.190103402293 .
- ↑ a b E. Fischer: Representation of the aceto-bromoglucose . In: Chem. Ber. tape 49 , no. 1 , 1916, p. 584-585 , doi : 10.1002 / cber.19160490162 .
- ↑ a b E. Fischer, E. Frankland Armstrong: About the isomeric acetohalogen derivatives of grape sugar and the synthesis of glucosides . In: Chem. Ber. tape 34 , no. 2 , 1901, p. 2885-2900 , doi : 10.1002 / cber.190103402251 .
- ↑ JK Dale: Preparation of bromoacetylglucose and certain other bromoacetyl sugars . In: J. Am. Chem. Soc. tape 38 , no. 10 , 1916, p. 2187–2188 , doi : 10.1021 / yes.02267a030 .
- ↑ CE Redemann, C. Niemann: Acetobromoglucose [2,3,4,6-Tetraacetyl-α-d-glucopyranosyl bromide] In: Organic Syntheses . 22, 1942, p. 1, doi : 10.15227 / orgsyn.022.0001 ; Coll. Vol. 3, 1955, p. 11 ( PDF ).
- ^ RU Lemieux: Methods in Carbohydrate Chemistry, Vol. 2, Reactions of Carbohydrates . Ed .: RL Whistler, ML Wolfrom, JN BeMiller. Academic Press Inc., New York 1963, pp. 221-222 .
- ↑ K. Mohri et al .: Synthesis of glycosylcurcuminoids . In: Chem. Pharm. Bull. Volume 51 , no. 11 , 2003, p. 1268-1272 , doi : 10.1248 / cbp.51.1268 .
- ↑ E. Fischer, K. Delbrück: Synthesis of new disaccharides of the trehalose type . In: Chem. Ber. tape 42 , no. 2 , 1909, p. 2776-2785 , doi : 10.1002 / cber.190904202203 .
- ^ CM McCloskey, GH Coleman: β-d-glucose-2,3,4,6-tetraacetate (D-glucose, β-2,3,4,6-tetraacetyl-) In: Organic Syntheses . 25, 1945, p. 53, doi : 10.15227 / orgsyn.025.0053 ; Coll. Vol. 3, 1955, p. 434 ( PDF ).
- ↑ B. Giese, J. Dupuis, M. Nix: 1-Deoxy-2,3,4,6-tetra-O-acetyl-1- (2-cyanoethyl) -α- D- glucopyranose In: Organic Syntheses . 65, 1987, p. 236, doi : 10.15227 / orgsyn.065.0236 ( PDF ).
- ↑ Patent US4751291 : Process for the preparation of glycosyl fluorides protected on the oxygen. Applied on September 12, 1986 , published on June 14, 1988 , applicant: Hoechst AG, inventor: J. Thiem, H.-M. Deger, W. Fritsche-Lang, M. Kreuzer.
- ↑ M. Yokoyama: Methods of synthesis of glycosyl fluorides . In: Carbohyd. Res. Band 327 , no. 1-2 , 2000, pp. 5-14 , doi : 10.1016 / S0008-6215 (99) 00324-9 .
- ↑ K. Toshima: Glycosyl fluorides in glycosylations . In: Carbohyd. Res. Band 327 , no. 1-2 , 2000, pp. 15-26 , doi : 10.1016 / S0008-6215 (99) 00325-0 .
- ^ S. Pearson, W. Scarano, MH Stenzel: Micelles based on gold-glycopolymer complexes as new chemotherapy drug delivery agents . In: Chem. Commun. tape 48 , 2012, p. 4695-4697 , doi : 10.1039 / C2CC30510K .
- ↑ P. Konradsson, UE Udodong, B. Fraser-Reid: Iodonium promoted reactions of disarmed thioglycosides . In: Tetrahedron Lett. tape 31 , no. 30 , 1990, pp. 4313-4316 , doi : 10.1016 / S0040-4039 (99) 97609-3 .
- ↑ G. Lian, X. Zhang, B. Yu: Thioglycosides in Carbohydrate Research . In: Carbohyd. Res. Band 403 , 2015, p. 13-22 , doi : 10.1016 / j.carres.2014.06.009 .
- ^ RR Schmidt, J. Michel: Simple synthesis of α- and β- O- glycosylimidates; Production of glycosides and disaccharides . In: Angew. Chem. Band 92 , no. 9 , 1980, pp. 763-765 , doi : 10.1002 / anie.19800920933 .
- ↑ a b R.R. Schmidt: New methods for glycoside and oligosaccharide synthesis - are there alternatives to the Koenigs-Knorr method? In: Angew. Chem. Band 98 , no. 3 , 1986, pp. 213-236 , doi : 10.1002 / anie.19860980305 .
- ↑ B. Helferich, K.-F. Wedemeyer: For the representation of glucosides from acetobromoglucose . In: Liebigs Ann. Chem. Band 563 , no. 1 , 1949, p. 139-145 , doi : 10.1002 / jlac.19495630115 .
- ↑ Patent DE19709787A1 : Oligosaccharides and their derivatives as well as a chemo-enzymatic process for their production. Registered on March 11, 1997 , published on September 17, 1998 , applicant: Bayer AG, inventor: W.-D. Fessner, M. Petersen, A. Papadopoulos, G. Oßwald.
- ^ VY Joshi, MR Sawant: A convenient stereoselective synthesis of β- D -glucopyranosides . In: Ind. J. Chem. 45B, 2006, p. 461-465 ( res.in [PDF]).
- ↑ T. Shi et al .: Development of a kilogram-scale synthesis of salidroside and its analogs . In: Synth. Commun. tape 41 , no. 17 , 2011, p. 2594–2600 , doi : 10.1080 / 00397911.2010.515332 .
- ↑ C. Mannich: About arbutin and its synthesis . In: Arch. Pharm. Band 250 , no. 1 , 1912, p. 547-560 , doi : 10.1002 / ardp.19122500146 .
- ↑ K. Mohri et al .: Synthesis of glycosylcurcuminoids . In: Chem. Pharm. Bull. Volume 51 , no. 11 , 2003, p. 1268-1272 , doi : 10.1248 / cbp.51.1268 .
- ↑ S. Hanessian, J. Banoub: Chemistry of the glycosidic linkage. An efficient synthesis of 1,2-trans-disaccharides . In: Carbohydr. Res. Band 53 , 1977, pp. C13-C16 , doi : 10.1016 / S0008-6215 (00) 85468-3 .
- ↑ a b H. Paulsen: Advances in the selective chemical synthesis of complex oligosaccharides . In: Angew. Chem. Band 94 , no. 3 , 1982, pp. 184-201 , doi : 10.1002 / anie.19820940304 .
- ^ RU Lemieux, KB Hendriks, RV Stick, K. James: Halide ion catalyzed glycosidation reactions. Syntheses of .alpha.-linked disaccharides . In: J. Am. Chem. Soc. tape 97 , no. 14 , 1975, p. 4056-4062 , doi : 10.1021 / ja00847a032 .