Silver trifluoromethanesulfonate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
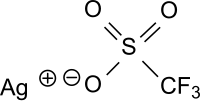
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Silver trifluoromethanesulfonate | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | CAgF 3 O 3 S | |||||||||||||||
| Brief description |
White to slightly pale yellow crystal powder or beige crystalline substance |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 256.94 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
|
|||||||||||||||
| solubility |
Easily soluble in water, acetone , ethanol and diethyl ether , soluble in many organic solvents, such as. B. acetonitrile , but also in benzene |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Silver trifluoromethanesulfonate (silver triflate, AgOTf) is the silver salt of trifluoromethanesulfonic acid with the formula CF 3 SO 3 - Ag + , which is found in water, but also in non-polar solvents, such as. B. is soluble in benzene. Silver triflate is a versatile reagent and a catalyst for acylations , cyclizations, or Friedel-Crafts acylations and Friedel-Crafts alkylations .
Occurrence and representation
An early route of preparation starts from the barium salt of trifluoromethanesulfonic acid (TfOH), from which the free TfOH is formed with dilute sulfuric acid , which is then neutralized with silver carbonate Ag 2 CO 3 .
The silver triflate is obtained in a yield of 95% and can be recrystallized from benzene / tetrachloromethane or ether / tetrachloromethane for purification .
In a variant improved by George Whitesides , dilute TfOH is reacted with silver (I) oxide Ag 2 O, which produces AgOTf in 98% yield.
properties
Silver trifluoromethanesulfonate is a white to beige, crystalline, odorless, light-sensitive and hygroscopic solid that is soluble in water and many organic solvents.
Applications
Activation of alkyl halides and Grignard compounds with silver triflate
Of silver trifluoromethanesulfonate and methyl iodide which is methylation agent Methyltrifluormethylsulfonat (methyl triflate, MeOTf) accessible in 69% yield.
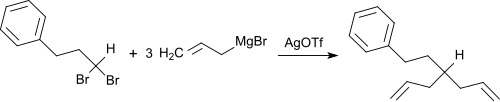
Silver triflate reacts with primary Grignard compounds or lithium organyls under mild conditions and with good yields with CC linkage. The reaction can be carried out using α, ω-Di-Grignard compounds - starting z. B. of cis-1,2-bis (chloromethyl) cyclohexane - use to represent carbocyclic four-, five- or six-membered rings.
The detachment of halide ions from alkyl halides is catalyzed by silver triflate, which z. B. can be used for the reaction of gem -dibromoalkanes with Grignard compounds with allylation or benzylation .
Bromination , Friedel-Crafts and sulfonation reactions with silver triflate
In the 1970s, Franz Effenberger's working group developed several different applications for silver trifluoromethanesulfonate.
Silver triflate catalyzes the bromination of electron-poor aromatics, such as, for example, trifluoromethylsulfonyl bromide CF 3 SO 2 OBr with bromine . B. 1,3-dinitrobenzene in concentrated sulfuric acid to 1-bromo-3,5-dinitrobenzene in 71% yield.
Silver trifluoromethanesulfonate reacts with aromatic carboxylic acid halides to form the mixed anhydrides which, without an additional catalyst, form aromatic ketones with aromatics in a Friedel-Crafts acylation.
Thus, with benzoyl chloride as carboxylic acid chloride and silver triflate, the mixed anhydride is formed in 90% yield, which forms benzophenone in 90% yield with benzene .
With alkyl - Sulfonsäurebromiden and silver triflate analogously to the corresponding mixed alkyl sulfonic anhydrides are accessible.
From ethylsulfonyl bromide Et-SO 2 Br (from ethyl bromide , magnesium and sulfur dioxide ) the mixed sulfonic acid anhydride is formed in 83% yield on reaction with silver triflate, which reacts with benzene to form ethylphenyl sulfone without the addition of a catalyst.
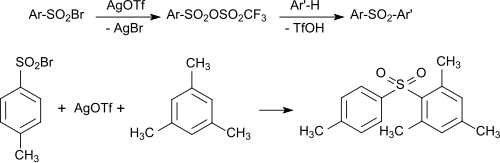
With silver triflate and aryl - Sulfonsäurebromiden mixed aryl sulfonic acid anhydrides formed may also generated in a one-pot process directly with aromatics to the corresponding diaryl sulfones are implemented.
Etherifications and esterifications with silver triflate
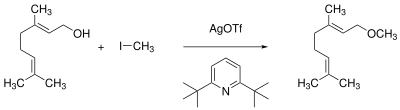
In the presence of non-nucleophilic amine bases, such as. B. 2,6-Di-tert-butylpyridine , even sensitive alcohols react with silver triflate and primary alkyl halides to form the corresponding alkyl ethers .
Geraniol is thus obtained from the terpene alcohol - presumably with formation of the alkylating agent methyl trifluoromethylsulfonate - with a three-fold excess of the reagents geraniol methyl ether in 95% yield.
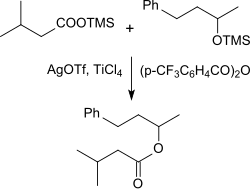
Silylated carboxylic acids can be reacted with silver triflate and catalytic amounts of the Lewis acid titanium tetrachloride with silylated alcohols in the presence of 4-trifluoromethylbenzoic anhydride to give the corresponding esters in very high yields.
Thus forming trimethylsilyl - isovaleric acid with silver triflate, the mixed carboxylic acid-trifluoromethanesulphonic acid anhydride which reacts with silylated 4-phenyl-2-butanol in 94% yield to give the ester.
Silver triflate catalyzes even in 1 mol percent addition - by reaction with acetic anhydride and intermediate formation of the mixed anhydride CH 3 CO-O-SO 2 CF 3 - in a smooth reaction (60 ° C, 1-20 min reaction time) the formation of the corresponding acetylation products of a series of alcohols, thiols , phenols and amines in practically quantitative yields (96–98%).
Lactonizations with silver triflate
The intramolecular addition of hydroxyl groups and carboxy groups to double bonds with the formation of five- (γ-) and / or six- (δ-) membered cyclic ethers or lactones is catalyzed by silver trifluoromethanesulfonate with very high yields.
Even unsaturated fatty acids can be cyclized intramolecularly with silver triflate at 130–160 ° C in useful yields (51–71%) with shifting of the double bond and selective formation of five-membered (γ-) lactones.
Glycosylation with silver triflate
A modification of the current Koenigs-Knorr method for the construction of disaccharides from the glycosyl donor 2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl bromide (acetobromoglucose) uses silver trifluoromethanesulfonate in equimolar amounts as a promoter and tetramethylurea as a proton acceptor . The process is characterized by a simplified process management as well as high anomer purity and yields of the products.
With silyl enol ethers, such as. B. 1-phenyl-1-trimethylsiloxyethene (acetophenonenol trimethylsilyl ether), which is easily accessible from acetophenone , triethylamine , chlorotrimethylsilane and sodium iodide in acetonitrile , react glycosyl halides, such as. B. 2,3,4,6- Tetra- O -benzyl-α-D-glucopyranosyl chloride in high yields to the corresponding β-glucoside.
The O - glycosyl trichloroacetimidates described by Richard R. Schmidt as versatile glycosyl donors can also be activated by means of silver triflate.
In combination with iodine chloride , silver trifluoromethanesulfonate catalyzes the glycosylation of thioglycosides to form O- glycosides in useful to good (46–82%) yields.
literature
- TH Black: Handbook of Reagents for Organic Synthesis: Reagents for Heteroarenes Functionalization . Ed .: AB Charette. Wiley, 2015, ISBN 978-1-118-72659-4 , pp. 636-648 .
Individual evidence
- ↑ Entry on Silver Trifluoromethanesulfonate at TCI Europe, accessed on February 18, 2017.
- ↑ a b c d data sheet Silver trifluoromethanesulfonate from Sigma-Aldrich , accessed on February 18, 2017 ( PDF ).
- ↑ Data sheet silver trifluoromethanesulfonate for synthesis (PDF) from Merck , accessed on February 18, 2017.
- ↑ a b c data sheet Silver trifluoromethane from AlfaAesar, accessed on February 18, 2017 ( PDF )(JavaScript required) .
- ↑ a b T.H. Black, KA Stubbs, RV Stick, J.-M. Weibel, P. Pale, CL Ladd: Silver (I) Trifluoromethanesulfonate . In: e-EROS Encyclopedia of Reagents for Organic Synthesis . 2015, doi : 10.1002 / 047084289X.rs032.pub4 .
- ↑ a b c T. Gramstadt, RN Haszeldine: 33. Perfluoroalkyl derivatives of sulfur. Part IV. Perfluoroalkanesulphonic acids . In: J. Chem. Soc. 1956, p. 173-180 , doi : 10.1039 / JR9560000173 .
- ↑ a b c R.N. Haszeldine, JM Kidd: Perfluoroalkyl derivatives of sulfur. Part I. Trifluoromethanesulphonic acid . In: J. Chem. Soc. 1954, p. 4228-4232 , doi : 10.1039 / JR9540004228 .
- ↑ a b R. Das, D. Chakraborty: Silver triflate catalyzed acetylation of alcohols, thiols, phenols, and amines . In: Synthesis . 2011, p. 1621-1625 , doi : 10.1055 / s-0030-1259999 .
- ↑ a b c G.M. Whitesides, FD Gutowski: Reaction of α, ω- di-Grignard reagents with silver (I) salts form carbocyclic rings . In: J. Org. Chem. Band 41 , no. 17 , 1976, p. 2882-2885 , doi : 10.1021 / ja00879a019 .
- ↑ T. Gramstadt, RN Hazeldine: 806. Perfluoroalkyl derivatives of sulfur. Part VII. Alkyl trifluoromethanesulphonates as alkylating agents, trifluoromethanesulphonic anhydride as a promoter for esterification, and some reactions of trifluoromethanesulphonic acid . In: J. Chem. Soc. 1957, p. 4069-4079 , doi : 10.1039 / JR9570004069 .
- ↑ Y. Mitamura, H. Someya, H. Yorimitsu, K. Oshima: Silver-catalyzed diallylation and dibenzylation of gem-dibromoalkanes with Grignard reagents . In: Synlett . 2010, p. 309-312 , doi : 10.1055 / s-0029-1219168 .
- ↑ K. Huthmacher, F. Effenberger: New reactive bromination reagents . In: Synthesis . tape 9 , 1978, p. 693-694 , doi : 10.1055 / s-1978-24861 .
- ↑ F. Effenberger, G. Epple: Trifluoromethanesulfonic acid carboxylic acid anhydrides, highly effective acylating agents . In: Angew. Chem. Band 84 , no. 7 , 1972, p. 294-295 , doi : 10.1002 / anie.19720840710 .
- ↑ F. Effenberger, K. Huthmacher: Presentation and reactions of trifluoromethanesulfonic acid sulfonic acid anhydrides . In: Angew. Chem. Band 86 , no. 11 , 1974, p. 409-410 , doi : 10.1002 / anie.1974086116 .
- ↑ G. Geiseler, R. Kuschmiers: The vibration spectra of methane and ethane sulfohalides . In: Chem. Ber. tape 93 , no. 9 , 1960, pp. 2014–2047 , doi : 10.1002 / cber.19600930920 .
- ↑ F. Effenberger, K. Huthmacher: Presentation and reactions of arylsulfonic acid trifluoromethanesulfonic acid anhydrides . In: Chem. Ber. tape 109 , no. 6 , 1976, p. 2315-2326 , doi : 10.1002 / cber.19761090636 .
- ↑ RM Burk, TS Gac, MB Roof: A mild procedure for etherification of alcohols with primary alkyl halides in the presence of silver triflate . In: Tetrahedron Lett. tape 35 , no. 44 , 1994, pp. 8111-8112 , doi : 10.1016 / 0040-4039 (94) 88256-8 .
- ↑ T. Mukaiyama, I. Shiina, M. Miyashita: An efficient method for the preparation of carboxylic esters via mixed anhydride by the promotion of a catalytic amount of Lewis acid . In: Chem. Lett. tape 21 , no. 4 , 1992, pp. 625-628 , doi : 10.1246 / cl.1992.625 .
- ↑ C.-G. Yang, NW Reich, Z. Shi, C. He: Intramolecular additions of alcohols and carboxylic acids to inert olefins catalyzed by silver (I) triflate . In: Org. Lett. tape 7 , no. 21 , 2005, p. 4553-4556 , doi : 10.1021 / ol51065f .
- ↑ LJ Goossen, DM Ohlmann, D. Dierker: Silvertriflate-catylyzed synthesis of γ-lactones from fatty acids . In: Green Chem. Band 12 , 2010, p. 197-200 , doi : 10.1039 / B916853B .
- ↑ S. Hanessian, J. Banoub: Chemistry of the glycosidic linkage. An efficient synthesis of 1,2-trans-disaccharides . In: Carbohydr. Res. Band 53 , no. 1 , 1977, pp. C13-C16 , doi : 10.1016 / S0008-6215 (00) 85468-3 .
- ↑ T. Mukaiyama, K. Narasaka: 3-Hydroxy-3-methyl-1-phenyl-1-butanone by crossed aldol reaction In: Organic Syntheses . 65, 1987, p. 2, doi : 10.15227 / orgsyn.065.0006 ; Coll. Vol. 8, 1993, p. 210 ( PDF ).
- ↑ P. Allevi, M. Anastasia, P. Ciuffreda, A. Fiecchi, A. Scala: C-Glucopyranosyl derivatives from readily available 2,3,4,6-tetra- O -benzyl-α-D-glucopyranosyl chloride . In: Chem. Commun. No. 2 , 1987, pp. 101-102 , doi : 10.1039 / C39870000101 .
- ↑ RR Schmidt: New methods for glycoside and oligosaccharide synthesis - are there alternatives to the Koenigs-Knorr method? In: Angew. Chem. Band 98 , no. 3 , 1986, pp. 213-236 , doi : 10.1002 / anie.19860980305 .
- ^ SP Douglas, DM Whitfield, JJ Krepinsky: Silver trifluoromethanesulfonate (triflate) activation of trichloroacetimidates in glycosylation reactions . In: J. Carbohydr. Chem. Band 12 , no. 1 , 1993, p. 131-136 , doi : 10.1080 / 07328309308018547 .
- ↑ G. Wei, G. Gu, Y. Du: Silver triflate. A mild alternative catalyst for glycosylation conditions using trichloroacetimidates as glycosyl donors . In: J. Carbohydr. Chem. Band 22 , no. 6 , 2003, p. 385-393 , doi : 10.1081 / CAR-120025325 .
- ↑ T. Ercegovic, A. Meijer, G. Mangnusson, U. Ellervik: Iodine monochloride / silver trifluoromethanesulfonate (ICl / AgOTf) as a convenient promoter system for O-glycoside synthesis . In: Org. Lett. tape 3 , no. 6 , 2001, p. 913-915 , doi : 10.1021 / ol015547c .


![Synthesis of bicyclo [4.2.0] octane](https://upload.wikimedia.org/wikipedia/commons/thumb/7/7a/Synthese_von_Bicyclo%284.2.0%29octan.svg/400px-Synthese_von_Bicyclo%284.2.0%29octan.svg.png)