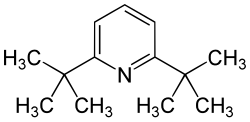
2,6-di- tert- butylpyridine
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 2,6-di- tert- butylpyridine | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 13 H 21 N | |||||||||||||||
| Brief description |
colorless liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 191.31 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.852 g cm −3 (25 ° C) |
|||||||||||||||
| Melting point |
2 ° C |
|||||||||||||||
| boiling point |
100–101 ° C (31 hPa ) |
|||||||||||||||
| pK s value |
3.58 |
|||||||||||||||
| solubility | ||||||||||||||||
| Refractive index |
1.473 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
2,6-Di- tert- butylpyridine is an organic compound that belongs to the heterocycles (more precisely: heteroaromatic compounds ). It consists of a pyridine ring with two tert-butyl radicals in the 2- and 6-positions .
Extraction and presentation
2,6-Di- tert- butylpyridine can be obtained by reacting 2,6-di- tert- butylpyrylium perchlorate with ammonium chloride. It can also be obtained by reacting 2- tert -butylpyridine with tert -butyllithium .
properties
2,6-Di- tert- butylpyridine is a colorless liquid. It is a weak base and proton catcher.
use
2,6-Di- tert- butylpyridine is used in research to study the polymerization of isobutylene and for the α- enolization of aldehydes , which lead to 1,4-dicarbonyl systems.
Individual evidence
- ↑ a b c d e f g h i j data sheet 2,6-di-tert-butylpyridine, ≥97% from Sigma-Aldrich , accessed on July 28, 2017 ( PDF ).
- ^ Advances in Heterocyclic Chemistry . Academic Press, 1988, ISBN 978-0-08-057630-5 , pp. 177 ( limited preview in Google Book search).
- ↑ a b Data sheet 2,6-Di-tert-butylpyridine, 97 +% from AlfaAesar, accessed on July 28, 2017 ( PDF )(JavaScript required) .
- ^ Pyridine derivatives, especially 2,6-di-tert-butylpyridine, labeled with nitrogen-15 . In: Journal of Labeled Compounds and Radiopharmaceuticals . tape 56 , no. 12 , 2013, p. 637-638 , doi : 10.1002 / jlcr.3071 .
- ↑ Herbert C. Brown, Bernard Kanner: Preparation and Reactions of 2,6-Di-t-butylpyridine and Related Hindered Bases. A Case of Steric Hindrance toward the Proton. In: Journal of the American Chemical Society . 88, 1966, pp. 986-992, doi : 10.1021 / ja00957a023 .
