Diamines
| Technically important diamines (selection) |
|
General structure of a diamine. The primary amino groups (NH 2 ) are marked in blue , R is a two-bonded organic radical (e.g. a para - phenylene group ). |
|
Hexamethylene-1,6-diamine |
 2,4-diaminotoluene |
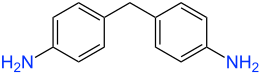 Diphenylmethane-4,4'-diamine |
 Isophoronediamine |
Diamines are a group of substances in organic chemistry . They are aliphatic or aromatic compounds that contain two amino groups (NH 2 ).
properties
In contrast to aromatic diamines - such as B. Phenylenediamine - many aliphatic diamines are soluble in water. The solutions are alkaline. Diamines form salts with acids. So z. B. by reacting diamine 1 (R = double-bonded organic residue, e.g. 1,2-ethylene or phenylene ) with hydrochloric acid dihydrochloride 2 , which are usually readily water-soluble:
use
Diamines are used as
- Stabilizers of melamine resins ,
- Additives to epoxy resins ,
- for the synthesis of dyes ,
- for the synthesis of medicinal substances ,
- for the synthesis of diisocyanates ,
- Raw material for polyamides ,
- Ligands in complex chemistry etc.
Diisocyanates
Diisocyanates are produced from diamines by reaction with phosgene (COCl 2 ):
The course of the reaction corresponds to the reaction of primary amines with phosgene. Diisocyanates are technically important raw materials for the production of polyurethanes (e.g. " construction foam ").
Individual evidence
- ↑ a b Otto Albrecht Neumüller (Editor): Römpp Chemie Lexikon , 8th edition, Frank'sche publishing firm, Stuttgart 1983, ISBN 3-440-04513-7 , S. 926th
- ↑ Otto-Albrecht Neumüller (editor): Römpps Chemie Lexikon , 8th edition, Frank'sche Verlagshandlung, Stuttgart 1983, ISBN 3-440-04513-7 , p. 961.
- ↑ Joachim Buddrus, Bernd Schmidt: Basics of Organic Chemistry , 5th edition, de Gruyter Verlag, Berlin 2015, ISBN 978-3-11-030559-3 , pp. 682–683.
