Caprolactam
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Caprolactam | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 6 H 11 NO | |||||||||||||||
| Brief description |
white scales |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 113.16 g · mol -1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.02 g cm −3 (75 ° C) |
|||||||||||||||
| Melting point |
70 ° C |
|||||||||||||||
| boiling point |
270 ° C |
|||||||||||||||
| solubility |
good in water (4650 g l −1 at 20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| MAK |
|
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Caprolactam , more precisely ε- caprolactam , is an economically important lactam that is produced annually on a megaton scale. It is used as a raw material for the production of polyamide 6 (Perlon).
history
Caprolactam was first produced in 1899 by Siegmund Gabriel and Theodor A. Maass through the cyclization of ε-aminocaproic acid. One year later, the later Nobel Prize winner Otto Wallach discovered the synthesis, which is still the most important to this day, via the Beckmann rearrangement of cyclohexanone oxime. An economic breakthrough came with the discovery of the reaction of caprolactam to polycaprolactam by Paul Schlack ( IG Farben ) in 1938. In 2016, the production volume of caprolactam was approximately 5.5 million tons.
Manufacture of caprolactam
Numerous different manufacturing routes are known for caprolactam. All commercial manufacturing processes are currently based on benzene from BTEX and use the Beckmann rearrangement . The remainder is enormous amounts of ammonium sulphate - 40 to 50% of the ammonium sulphate produced globally is created during the production of caprolactam. The processes can be divided into two groups.
Manufactured via cyclohexanone
Almost all commercial manufacturing processes use cyclohexanone as an intermediate stage - 98% of the caprolactam is manufactured this way. All of them can be roughly divided into three steps:
- Cyclohexanone is made from benzene
- The cyclohexanone is by means of hydroxylamine in cyclohexanone converted
- The cyclohexanone oxime reacts in the presence of sulfuric acid in a Beckmann rearrangement to form ε-caprolactam
There are several reaction pathways that are practiced for the production of cyclohexanone from benzene. For example, benzene can first be catalytically hydrogenated and then oxidized.
For the production of the hydroxylamine in the second process step, variants of the Raschig process are traditionally used. However, since large quantities of the waste product ammonium sulfate are produced here (up to 2.7 tons per ton of oxime), other more atom-economical methods have also developed.
Ammonium sulphate is also produced as a waste process in the third process step, so that the total amount of by-products is 2 to 4.5 tons per ton of caprolactam. This reaction step is strongly exothermic, the heat of reaction is −105.1 kJ mol −1 . Therefore, continuous cooling is required.
Photooximation
This production does not require cyclohexanone as an intermediate and can also be divided into three steps:
- Cyclohexane is produced from benzene in a catalytic hydrogenation
- Cyclohexane is converted to cyclohexanone oxime (more precisely: to cyclohexanone oxime hydrochloride) using nitrosyl chloride and hydrogen chloride
- Finally, analogous to the other processes, the Beckmann rearrangement of cyclohexanone oxime to ε-caprolactam in sulfuric acid follows
In this process, only 1.55 tons of ammonium sulfate are produced per ton of caprolactam. However, expensive reactors are e.g. B. made of titanium, because intermediate products such as nitrosyl chloride are very corrosive in this process.
Recycling of polycaprolactam
Caprolactam can also be obtained from polycaprolactam by recycling. Such methods are well known and established. To do this, the polymer must first be depolymerized. This usually takes place in a boiler with superheated steam, bases and a catalyst such as phosphoric acid at atmospheric pressure. It is also possible to work at high pressures without a catalyst. The resulting mixture of substances is then concentrated and unwanted components are converted into by oxidizing agents . The crude caprolactam is then purified by distillation. The caprolactam produced in this way has new product quality.
Production from renewable raw materials
Numerous efforts have been made to produce caprolactam from renewable raw materials. There are three different approaches:
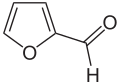
One possibility is to continue using the current processes and to produce the starting material benzene from biomass by catalytic pyrolysis . The second possibility is to find new chemical synthesis routes for the product caprolactam that are based on raw materials that can easily be produced using bio-based methods. Some such routes are now known. For example, caprolactam can be produced very selectively and in just three steps from bio-based hydroxymethylfurfural . The last option is the fermentative production of caprolactam precursors, which are then chemically processed in the “classic” manner. There are, for example, synthesis routes based on L- lysine or 1,3-butadiene , both of which can be produced by fermentation.
Currently (2017) no bio-based process is economically competitive, however, many such synthesis routes are expected to be implemented in the following decades.
- Structural formulas of some precursors of caprolactam that can be produced bio-based
properties
Physical Properties
At room temperature, ε-caprolactam forms white crystals that are readily soluble in water, are hygroscopic and have a characteristic odor. It is therefore usually stored at temperatures above 80 ° C. until it is used.
Chemical properties
Caprolactam is soluble in polar and aromatic solvents and not very soluble in long-chain aliphatic hydrocarbons. In the presence of water it hydrolyzes at 260–270 ° C to ε-aminocaproic acid . When caprolactam is heated to a higher degree in the presence of oxygen, it decomposes with the formation of u. a. Ammonia and nitrogen oxides ; if you abstain from oxygen, it is heat-stable.
Of outstanding importance is the possibility of polymer formation, in which polycaprolactam (polyamide 6) is formed. This usually takes place via ring-opening polymerization . In addition, aminocaprolactam can be produced from caprolactam by nitration and subsequent reduction . In addition, caprolactam enters into all the typical reactions of lactams .
use
In industry, caprolactam is used almost exclusively for the production of polycaprolactam (polyamide 6), which is also known under the brand name Perlon. From this, for example, simple textile fibers and foils, but also high-quality and resistant yarns for technical applications are made.
Smaller amounts of caprolacatam are also used for chemical synthesis. For example, the amino acid L- lysine can be produced from caprolactam via the intermediate stage of aminocaprolactam .
literature
- Johan Tinge, Marijke Groothaert, Hans op het Veld, Josef Ritz, Hugo Fuchs, Heinz Kieczka & William C. Moran: Caprolactam . In: Ullmann's Encyclopedia of industrial chemistry . Wiley-VCH, Weinheim 2018, doi : 10.1002 / 14356007.a05_031.pub3 .
- Gerd Dahlhoff, John PM Niederer & Wolfgang F. Hoelderich: ϵ-Caprolactam: new by-product free synthesis routes . In: Catalysis Reviews: Science and Engineering . tape 43 , no. 4 , 2001, p. 381-441 , doi : 10.1081 / CR-120001808 .
Individual evidence
- ↑ a b c d e f g h Entry on epsilon-caprolactam in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ↑ Entry on ε-caprolactam in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 105-60-2 or ε-caprolactam ), accessed on February 20, 2020.
- ↑ Siegmund Gabriel & Theodor A. Maass: Ueber ε ‐ Amidocaproäure . In: Reports of the German Chemical Society . tape 32 , 1899, pp. 1266-1272 , doi : 10.1002 / cber.189903201205 .
- ↑ caprolactam. September 19, 2016, accessed October 24, 2019 .
- ↑ Kozyro, AA; Marachuk, LI; Krasulin, AP; Yursha, IA; Kabo, G.Ya .: Thermodynamic properties of cyclohexanone oxime and ε-caprolactam in J. Appl. Chem. USSR, 62 (1989) 547-550.