3-amino-2-azepanone
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||
| ( R ) - (+) - shape (left) and ( S ) - (-) - shape (right) | |||||||||||||
| General | |||||||||||||
| Surname | 3-amino-2-azepanone | ||||||||||||
| other names |
|
||||||||||||
| Molecular formula | C 6 H 12 N 2 O | ||||||||||||
| Brief description |
whitish to pale yellow crystal powder or long white needles |
||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | |||||||||||||
| Physical state |
firmly |
||||||||||||
| Melting point | |||||||||||||
| boiling point | |||||||||||||
| solubility |
soluble in water, slightly soluble in ethanol and insoluble in organic solvents |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
3-Amino-2-azepanone ( DL- aminocaprolactam ) is the ε- lactam of the amino acid L- lysine , which in most manufacturing processes is a racemate [1: 1 mixture of ( R ) - (+) - and ( S ) - (-) - aminocaprolactam] is obtained.
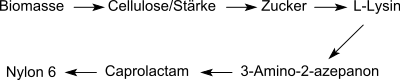
While DL -Aminocaprolactam previously as a chemical feedstock for the nowadays produced exclusively by means of biotechnology L served -lysine, in the current literature, α-amino-ε-caprolactam is used as an intermediate in the synthesis of ε-caprolactam and nylon-6 from biomass discussed.
presentation
DL -aminocaprolactam was already synthesized in 1905 by Emil Fischer as "lysine anhydride" when DL -lysine methyl ester was heated in substance with sodium methoxide to 100 ° C, but it was thought to be the disubstituted diketopiperazine formed from two molecules of lysine .
DW Adamson found in 1943 that Fischer's “lysine anhydride” consisted of 40% of what he called “dl-3-aminohomopiperidone” DL aminocaprolactam (formed from a lysine molecule), from which E. Fischer already made pure DL aminocaprolactam -Hydrochloride had shown.
Starting from the ε-caprolactam, which is inexpensive in large quantities, a multi-stage process starting with the triple chlorination with phosphorus oxychloride , phosphorus pentachloride and sulfuryl chloride leads to α, α-dichloro-ε-caprolactim chloride , which is converted into α, α with water -Dichloro-ε-caprolactam is decomposed (75-80% yield). Hydrogenation in the presence of Raney nickel in methanol / triethanolamine gives DL -α-chloro ε-caprolactam (85-90% yield), which reacts with sodium azide to form DL -α-azido-ε-caprolactam (80-85% yield). Catalytic hydrogenation of the azide with Raney nickel produces the target product DL -α-amino-ε-caprolactam (90–94% yield).
The overall yield of 46 to 58% across all stages makes this process economically unattractive.
The separation is carried out of the resulting racemate by formation of diastereomeric salt with L - (-) - pyrrolidonecarboxylic acid in ethanol , from which the L , L spontaneously crystallized salt, and subsequent separation of the isomerically pure salt in 1,4-dioxane by bubbling hydrogen chloride gas .
Another synthetic route, starting from ε-caprolactam, involves the reaction with phosgene to form 2-chlorazacyclo-2,3-hepten-1-carbochloride, which is first nitrated with nitrating acid to give 3-nitro-2-azepanone-1-carbochloride, and reacts with water to form 3-nitro-ε-caprolactam, splitting off carbon dioxide and chloride ions. The hydrogenation of the racemic 3-nitrocaprolactam obtained to give DL -3-amino-ε-caprolactam takes place practically quantitatively at about 100 ° C. and about 150 atm of hydrogen pressure over a palladium - activated carbon catalyst.
The cleavage of the racemic DL -3-amino-ε-caprolactam can be carried out by salt formation with L -5-pyrrolidone-2-carboxylic acid.
Phosgene or phosphorus oxychloride or phosphorus pentachloride and dimethylformamide react to form the so-called Vilsmeier reagent , which forms 1-formyl-ε-caprolactam with ε-caprolactam in chloroform and is then reacted with nitrating acid to form 1-formyl-3-nitro-azacycloheptanone. The formyl group is split off by heating with water to give 3-nitrocaprolactam, which is then catalyzed with Raney nickel or palladium / activated carbon and hydrogenated to give DL -3-aminocaprolactam. With a chiral ruthenium catalyst , such as. B. Ru 2 Cl 4 (diop) 3 , racemic 3-nitrocaprolactam can be hydrogenated asymmetrically to 3-aminocaprolactam - but only with an enantiomeric excess ( ee ) of 39% - from which L- lysine dihydrochloride is obtained by acid hydrolysis .
A lactamization of lysine without racemization can be achieved by heating L- lysine hydrochloride in xylene with hexamethyldisilazane (HMDS) as a condensing agent for 48 hours in a one-pot reaction with intermediate formation of the trimethylsilyl-amino acid ester to ( S ) -α-amino-ε-caprolactam- Hydrochloride can be brought about in 82% yield.
When L- lysine is refluxed for 20 hours in toluene on a water separator with the addition of neutral aluminum oxide , 3-amino-2-azepanone is obtained in 71% yield, but the end product is 41% racemized.
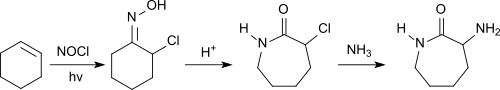
A combination of chemical synthesis and enzymatic cleavage is based on the petrochemical raw material cyclohexene , which reacts with nitrosyl chloride under light irradiation in the presence of hydrogen chloride to form 1-chlorocyclohexanone oxime .
After Beckmann rearrangement of the oxime to lactam, when the chlorine atom is replaced by an amino group by means of ammonia, DL -α-amino-ε-caprolactam is obtained, which is cleaved to L- lysine with the enzyme L -aminocaprolactam hydrolase . The unreacted D -α-amino-ε-caprolactam can be racemized into the DL -α-amino-ε-caprolactam by an α-amino-ε-caprolactam racemase .
The simplest and inexpensive chemical reaction variant appears to be the implementation of L- lysine hydrochloride and sodium hydroxide solution in equimolar amounts in higher-boiling alcohols, such as. B. 1-hexanol or 1,2-propanediol for approx. 8 hours under reflux and water separation, crystalline DL -α-amino-ε-caprolactam hydrochloride being obtained in approx. 75% yield after the addition of hydrochloric acid .
Deamination with hydroxylamine-O-sulfonic acid leads to ε-caprolactam in 75% yield. With this reaction sequence, ε-caprolactam, the monomer for the technical polymer nylon 6, is accessible from biomass.
As long as the price for the deamination reagent hydroxylamine- O- sulfonic acid in the last reaction stage is higher than that of the end product ε-caprolactam, commercial use of this multi-stage process is not to be expected.
properties
3-Amino-2-azepanone is a white to yellowish crystalline solid which is hygroscopic in substance and has a strongly basic reaction in aqueous solution. The amino compound is often called a more easily crystallizable and manageable hydrochloride ( DL -α-amino-ε-caprolactam hydrochloride, CAS # 29426-64-0) - e.g. B. precipitated from methanolic solution by introducing dry hydrogen chloride - isolated, which is "easily soluble" in water and easily soluble in methanol and ethanol .
Hydrochloric acid cleaves the lactam ring and rapidly decomposes 3-amino-2-azepanone to DL -lysine hydrochloride.
use
The addition of small amounts of α-amino-ε-caprolactam, together with equimolar amounts of dicarboxylic acids , such as. B. azelaic acid , in the ring-opening polymerization of caprolactam leads to (co) polyamide 6 types after significantly shorter reaction times, which can be post-condensed in the solid phase to give high molecular weight, branched, but m-cresol -soluble products with increased melt viscosity.
The synthesis and properties of oligomeric epoxide / amine adducts with the amines N -isopropylacrylamide (from the amino acid glycine ) or α-amino-ε-caprolactam (from lysine) and the diepoxide glycerol diglycidyl ether derived from biological precursors have recently been described.
The water-soluble and thermoresponsive oligomers show significant differences in curing time, viscosity and rigidity , especially in the presence of methylated cyclodextrins as solubilizers.
Before the triumphant advance of the biochemical synthesis of L- lysine by fermentation , 3-amino-2-azepanone was of certain interest as an intermediate stage in an industrial chemical synthesis of L- lysine - starting from ε-caprolactam from the petrochemical raw material cyclohexane .
The concept of the biorefinery is based on the substitution of intermediate products from petrochemical raw materials by analogues from biomass via enzymatic transformations in reverse of conventional synthetic chemistry: in this specific case, the conversion of L- lysine via the intermediate product 3-aminocaprolactam to ε-caprolactam as a monomer for the technical Plastic polyamide 6 . The synthesis routes proposed so far are not yet competitive with conventional synthesis.
Individual evidence
- ↑ a b c d data sheet 3-Amino-2-azepanone from Sigma-Aldrich , accessed on September 24, 2015 ( PDF ).
- ↑ a b Entry on DL-α-Amino-ε-caprolactam at TCI Europe, accessed on July 12, 2016.
- ↑ a b c d e f D.W. Adamson: 13. The anhydrides of basic amino acids . In: J. Chem. Soc. 1943, p. 39-40 , doi : 10.1039 / JR9430000039 .
- ↑ a b c Patent DE1115257 : Process for the production of D, L-α-amino-ε-caprolactam. Applied on October 19, 1961 , published on March 14, 1958 , applicant: JR Geigy A.-G., inventor: CM Brenner, H.-R. Rickenbacher.
- ↑ a b c Patent US8367819 : Synthesis of caprolactam from lysine. Filed July 11, 2011 , published February 5, 2013 , applicant: Board of Trustees of Michigan State University, inventor: JW Frost.
- ↑ a b E. Fischer, U. Suzuki: Synthesis of Polypeptiden. X. Polypeptides of diamino and oxyamino acids . In: Ber. German Chem. Ges. Volume 38 , no. 4 , 1905, pp. 4173-4196 , doi : 10.1002 / cber.190503804101 .
- ↑ Patent DE1194864 : Process for the production of L - (-) - α-amino-ε-caprolactam and its salts. Registered on March 14, 1958 , published on March 19, 1970 , applicant: JR Geigy AG, inventor: CM Brenner, H.-R. Rickenbacher.
- ↑ Patent US3080358 : Preparation of azacyclo-2,3-alkenes-2-chloro-N-carbochloride. Applied on September 18, 1958 , published March 5, 1963 , applicant: Stamicarbon NV, inventor: JH Ottenheym, JW Garritsen.
- ↑ Patent US3093634 : Preparation of α-Nitrolactams. Registered on September 10, 1959 , published on June 11, 1963 , Applicant: Stamicarbon NV, Inventor: JH Ottenheym, JPH von den Hoff.
- ↑ Patent US3096326 : Preparation of α-nitro-ε-caprolactam. Registered on February 19, 1962 , published on July 2, 1963 , applicant: Stamicarbon NV, inventor: J.de Haan, JPH von den Hoff.
- ↑ Patent US3048580 : Preparation of α-aminolactams by catalytic hydrogenation of α-nitrolactams. Registered on September 10, 1959 , published on August 7, 1962 , applicant: Stamicarbon NV, inventor: JH Ottenheym, PL Kerkhoffs.
- ↑ E. Breitmaier, G. Jung: Organic Chemistry: Fundamentals, classes of substances, reactions, concepts, molecular structure, 5th revised. Edition . Thieme , 2005, ISBN 3-13-541505-8 , pp. 782 , doi : 10.1016 / j.tet.2005.08.031 .
- ↑ Patent US3687940 : Process for the production of 3-amino-azacycloheptan-2-ones. Applied on June 24, 1970 , published on August 29, 1972 , applicant: Ciba-Geigy Corp., inventor: VR Foitl, W. Traber.
- ↑ Patent US3557093 : 1-Formyl-3-nitro-azacycloheptane-2-ones and process for their production. Applied on June 24, 1970 , published on August 29, 1972 , applicant: Ciba-Geigy Corp., inventor: VR Foitl, W. Traber.
- ↑ Patent EP0083332 : Asymmetric reduction of nitro-containing prochiral compounds. Applied December 20, 1982 , published July 6, 1983 , Applicant: Monsanto Co., Inventor: GL Bachman, MJ Sabacky.
- ↑ R. Pellegata, M. Pinza, G. Pifferi: An improved synthesis of γ-, δ-, and ε-lactams . In: Synthesis . 1978, p. 614-616 , doi : 10.1055 / s-1978-24834 .
- ↑ A. Bladé-Font: Facile synthesis of γ-, δ-, and ε-lactams by cyclodehydration of α-amino acids on alumina and silica gels . In: Tetrahedron Lett. tape 21 , no. 25 , 1980, pp. 2443-2446 , doi : 10.1016 / S0040-4039 (00) 93171-X .
- ↑ M. Ohno, N. Naruse, S. Torimitsu, M. Okamoto: Reactions of 2-Chlorocycloalkanone Oximes. I. Their Preparations and Conversion to 2-Alkoxy-, 2-Acyloxy- and 2-Alkylthiocycloalkanone Oximes . In: Bull. Chem. Soc. tape 39 , no. 6 , 1966, pp. 1119-1124 , doi : 10.1246 / bcsj.39.1119 .
- ↑ Y. Isumi, I. Chibata, T. Itoh: Production and use of amino acids . In: Angew. Chem. Band 90 , no. 3 , 1978, p. 187-194 , doi : 10.1002 / anie.19780900307 .
- ↑ Patent EP0288894 : Alpha-amino-epsilon-caprolactam modified polyamides. Registered on November 2, 1988 , published on September 25, 1991 , applicant: Bayer AG, inventor: Dr. Rolf-Volker Meyer, Dr. Rolf Dhein, Dr. Martin Wandel, Dr. Harald Selbeck, Dipl.-Ing. Friedrich Fahnler, Dr. Hans-Detlef Heinz, Dr. Peter-Rolf Müller.
- ↑ J. Fischer, H. Ritter: Oligomeric epoxide-amine adducts based on N-isopropylacrylamide and α-amino-ε-caprolactam: Solubility in the presence of cyclodextrin and curing properties . In: Beilstein J. Org. Chem. Volume 9 , 2013, p. 2803-2811 , doi : 10.3762 / bjoc.9.315 .