Azelaic acid
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
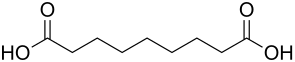
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Azelaic acid | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 9 H 16 O 4 | ||||||||||||||||||
| Brief description |
white, crystalline solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| ATC code | |||||||||||||||||||
| Drug class |
Acne medication |
||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 188.22 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.23 g cm −3 |
||||||||||||||||||
| Melting point |
98-102 ° C |
||||||||||||||||||
| boiling point | |||||||||||||||||||
| pK s value |
|
||||||||||||||||||
| solubility |
slightly soluble in water (2.4 g l −1 at 20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Azelaic acid (nonanedioic acid, 1,7-heptanedicarboxylic acid) is a chemical compound from the homologous series of dicarboxylic acids . Their salts and esters are called azelates .
properties
As a dibasic acid, it dissociates in water in two stages of protolysis . There are three polymorphic forms, which are divided into α, β and γ.
presentation
Azelaic acid can be made by oxidizing castor oil with potassium permanganate . First the castor oil is hydrolyzed to ricinoleic acid, which is then oxidized to azelaic acid.
The industrial production of azelaic acid is carried out by ozonolysis of oleic acid . By alkali melting of castor oil with NaOH, sebacic acid and 2-octanol (caprylic alcohol) are industrially produced via ricinoleic acid as an intermediate .
use
Azelaic acid is used as an active pharmaceutical ingredient in topical therapy for acne and rosacea . 20% cream and 15% gel preparations are used. Azelaic acid preparations require a prescription.
Various Di ester of azelaic acid, such as with 2-ethylhexanol ( diisooctyl azelate , DIOZ) with butanol ( dibutyl , DBZ) or octanol ( dioctyl azelate , DOZ), are used as plasticizers for plastics .
Trade names
Skinoren (D, A, CH), generic (A)
Individual evidence
- ↑ a b c Azelaic acid data sheet (PDF) from Merck , accessed March 30, 2010.
- ↑ a b Azelaic acid data sheet at AlfaAesar, accessed on March 30, 2010 ( PDF )(JavaScript required) .
- ↑ a b CRC Handbook of Tables for Organic Compound Identification , Third Edition, 1984, ISBN 0-8493-0303-6 .
- ↑ a b Entry on azelaic acid in the GESTIS substance database of the IFA , accessed on January 9, 2019(JavaScript required) .
- ↑ PM Babkov et al. Ukr. Fiz. Zh. (Russ Ed.) 1985.
- ↑ TW Abraham, R. Höfer, Lipid-Based Polymer Building Blocks and Polymers , In: K. Matyjaszewski, M. Möller (eds.): Polymer Science: A Comprehensive Reference, Vol 10, Polymers for a Sustainable Environment and Green Energy , JE McGrath, MA Hickner, R. Höfer (Vol. Edts.) Elsevier, Amsterdam, Oxford, Waltham (2012) pp. 15-58
- ↑ External identifiers or database links to diisooctyl azelate : CAS number: 26544-17-2, EC number: 247-774-8, ECHA InfoCard: 100.043.416 , PubChem : 66934 , Wikidata : Q72467323 .
- ↑ External identifiers from or database links to dibutylazelate : CAS number: 2917-73-9, EC number: 220-850-8, ECHA InfoCard: 100.018.956 , PubChem : 18016 , ChemSpider : 17020 , Wikidata : Q27290265 .
