Thermoresponsive Polymers
Thermoresponsive polymers are polymers that change their physical properties drastically and discontinuously with temperature. The term is mostly used when the property concerned is solubility in a particular solvent , but need not be limited to this. Thermoresponsive polymers belong to the class of stimuli-responsive materials, in contrast to sensitive materials, which only continuously adapt their properties to external conditions. They are also functional polymers .
Temperature-responsive polymer in the narrower sense exhibit in their temperature-composition diagram miscibility gap on. Depending on whether the miscibility gap occurs at high or low temperature, a lower or upper exists critical solution temperature ( engl. Lower or upper critical solution temperature , LCST or UCST abbreviated). After the common English abbreviation, the polymers in question are often referred to as LCST or UCST polymers for short.
The main focus of research is on polymers that exhibit thermal responsiveness in aqueous solution. Tissue engineering , chromatography , drug release and bioseparation are seen as promising fields of application . So far there are few commercial applications. One example are cell culture plates coated with LCST polymers.
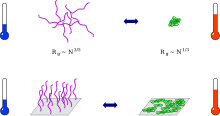
The ball-globule transition
Thermoresponsive polymers exist in solution as open-chain coils. At the phase separation temperature, these collapse into compact globules ( coil-to-globuli transition ). This process can be observed directly using methods of static and dynamic light scattering. The drop in viscosity can be followed indirectly . If there are no mechanisms in place to minimize the surface tension between the globules and the solvent, the globules aggregate , which initially manifests itself in increasing cloudiness of the solution and finally in the formation of visible particles.
The phase diagram of thermoresponsive polymers
The phase separation temperature (and thus also the cloud point) depends on the polymer concentration. Therefore, temperature-composition diagrams are used to show the thermoresponsive behavior over a wide range of concentrations. Phase separation takes place in a polymer-poor and a polymer-rich phase. In strictly binary mixtures, the composition of the coexisting phases could be determined by the formation of the conode (see critical solution temperature ). Since polymers generally have a molar mass distribution, this simple method can only be used to a limited extent.
In the course of the phase separation it can happen that the polymer-rich phase solidifies like glass before the equilibrium state is reached. This depends on the glass transition temperature for the particular composition of the mixture. It is useful to show the development of the glass transition temperatures in the phase diagram, although it is not a state of equilibrium. The point of intersection between the glass point curve and the cloud point curve is called the Berghmans point. In the case of UCST polymers, phase separation into two liquid phases takes place above the Berghmans point, below into a liquid, polymer-poor phase and a glass-like solidified polymer-rich phase. The inverse behavior is observed for LCST polymers.
thermodynamics
Polymers dissolve in a solvent when this reduces the Gibbs energy of the system, i. H. the change in Gibbs energy (ΔG) is negative. The well-known Legendre transformation of the Gibbs-Helmholtz equation shows that ΔG is composed of the enthalpy of mixing (ΔH) and entropy of mixing (ΔS).
If there were no interactions between the substances involved, there would be no enthalpy of mixing and the entropy of mixing would be ideal. The ideal entropy of mixing of several pure substances is always positive (the term - T · Δ S then negative) and Δ G would be negative for every mixing ratio. There would be complete miscibility. From this it follows that miscibility gaps must be explained with interactions between the components. In the case of a polymer solution, polymer-polymer, solvent-solvent, and polymer-solvent interactions must be considered. A model for the phenomenological description of polymer phase diagrams was developed by Flory and Huggins (see Flory-Huggins theory ).
From the Flory-Huggins theory, for example, it follows that the UCST (if present) increases with increasing molar mass and at the same time shifts into the solvent-rich zone. Whether a polymer exhibits LCST and / or UCST behavior can be deduced from the temperature dependence of the interaction parameter (see figure). It should be noted that the interaction parameter contains not only enthalpic elements, but also the non-ideal entropy of mixing (e.g. the very strong hydrophobic effect in aqueous solution). Since the interaction parameter contains both enthalpic and entropic elements, which in turn are composed of many individual components, the classical Flory-Huggins theory makes it difficult to draw conclusions about the molecular cause of miscibility gaps.
Applications
Bioseparation
Thermoresponsive polymers can be equipped with functional groups that specifically bind to certain biomolecules. These biomolecules can then be precipitated together with the polymer by a slight change in temperature. Isolation is possible by filtration or centrifugation.
Thermoresponsive surfaces in tissue engineering and chromatography
For some polymers it could be demonstrated that thermoresponsive behavior can be transferred to surfaces. For this purpose, the surface can either be coated with a polymer film or the polymer chains can be covalently bonded to the surface. The wetting of the surface with the solvent can be controlled by small changes in temperature. The behavior described can be used, for example, in tissue engineering , since the adhesion of cells to surfaces is highly dependent on the hydrophilicity / hydrophobicity of the surface. It is thus possible to detach cells from a correspondingly coated cell culture plate by means of a small change in temperature without the otherwise usual use of enzymes. Corresponding products are already commercially available.
The use of thermoresponsive polymers as stationary phases in liquid chromatography is also being investigated . The polarity of the stationary phase can be extremely influenced by a change in temperature, whereby the separating effect can be varied for different substance classes without changing the column.
Thermoresponsive gels
Three-dimensional polymer networks are insoluble in all solvents; they can only swell . Thermoresponsive polymers show a discontinuous course of the degree of swelling with temperature. At the volume phase transition temperature (VPTT) there is a strong change in the degree of swelling. Numerous research projects try to use this behavior for the temperature-induced release of active ingredients, since previously stored active ingredients can easily diffuse out of gel in the swollen state.
Characterization of thermoresponsive polymer solutions
Cloud point
The phase separation can be investigated experimentally simply by means of turbidimetry. There is no procedure for determining the cloud point that is equally useful for all systems. There is therefore no uniform definition. It is often defined as the temperature at which a first turbidity can be detected (onset), the temperature at the point of inflection in the transmission curve or the temperature at a defined transmission (e.g. 50%). The term for the temperature when the solution clears up is also undefined, as the term clearing point is already used for phase transitions in liquid crystals.
Hysteresis
The cloud points (or “clearing points”) when a thermoresponsive polymer solution is cooled down and heated up are not identical because it takes time to establish equilibrium. The temperature interval between the cloud points in the cooling and heating phases is called hysteresis. The cloud points are dependent on the cooling or heating rate used and the hysteresis decreases with decreasing rates. It is assumed that the hysteresis depends on the temperature, viscosity, glass transition temperature and the ability to form additional intra- and intermolecular bonds in the phase-separated state.
Other properties
The extent to which the polymer content of the two phases differs after separation is very important for potential applications. For most applications, a phase separation into pure polymer and pure solvent would be desirable, but this is not possible in practice. The course of the phase separation depends on the exact form of the phase diagram.
Example : From the phase diagram of a solution of polystyrene (molar mass 43,600 g / mol) in the solvent cyclohexane it can be deduced that with a total polymer concentration of 10% when cooling from 25 to 20 ° C, a low-polymer phase with approx. 1% polymer and a polymer-rich phase with approx. 30% polymer is formed.
A sharp phase transition is also desirable for many applications, which manifests itself in an abrupt drop in the transmission curve. The sharpness of the phase transition depends on the strength of the phase separation, but is also influenced by whether all the polymer chains present in the mixture have the same cloud point. This depends on the polymer end groups, the dispersity and, if necessary, on varying copolymer compositions.
Examples of thermoresponsive polymers
Thermal responsiveness in organic solvents
Due to the low entropy of mixing, miscibility gaps occur relatively frequently in polymer solutions. A large number of polymers are known which show UCST and / or LCST behavior in organic solvents. Examples of organic polymer solutions with UCST are polystyrene in cyclohexane, polyethylene in diphenyl ether or polymethyl methacrylate in acetonitrile. An LCST is found, for example, for the systems polypropylene in n- hexane, polystyrene in butyl acetate or polymethyl methacrylate in 2-propanone.
Thermoresponsivity in water
Polymer solutions that show thermoresponsiveness in water are of particular importance because the solvent water is cheap, safe and biologically relevant. In science, attempts have been made for a long time to make water-soluble thermoresponsive polymers useful for the release of active ingredients or intelligent materials in tissue engineering . Many polymers with LCST in water are known. The best researched is poly-N-isopropyl acrylamide . Further examples are hydroxypropyl cellulose , poly (vinyl caprolactam) and polyvinyl methyl ether.
A number of polymers produced on an industrial scale show both LCST and UCST behavior in water. However, the UCST is usually located in temperature ranges outside the 0–100 ° C spectrum and can therefore only be determined under extreme test conditions. Examples are polyethylene oxide, polyvinyl methyl ether and polyhydroxyethyl methacrylate. There are also examples of polymers that exhibit UCST behavior in the range between 0 and 100 ° C. However, there are large differences in the ionic strength at which UCST behavior can be observed. Some polyzwitterions show UCST behavior in pure water, but not in salty water. Polyacrylic acid, on the other hand, shows UCST behavior only at high ionic strengths. Examples of polymers which can show UCST behavior both in pure water and under physiological conditions are poly ( N- acrylglycine amide), urea-functionalized polymers and copolymers of acrylamide and acrylonitrile. For the determination of cloud points in pure water, however, the polymers must not contain any ionic groups.
In the examples mentioned, it should be noted that the UCST depends on the molar mass of the polymers. This is not necessarily the case with LCST, as shown for poly ( N -isopropyl acrylamide).
Individual evidence
- ^ Allan S. Hoffman, "Intelligent" Polymers in Medicine and Biotechnology , Artificial Organs, 1995, Volume 19, pp 458-467.
- ^ A b c Mark A. Ward, Theoni K. Georgiou, Thermoresponsive Polymers for Biomedical Applications , Polymers, 2011, Volume 3, pp. 1215-1242.
- ^ A b Irene Tan, Farnoosh Roohi, Maria-Magdalena Titirici, Thermoresponsive polymers in liquid chromatography . In: Analytical Methods , 2012, Volume 4, pp. 34-43.
- ↑ AK Bajpai, Sandeep K. Shukla, Smitha Bhanu, Sanjana Kankane, Responsive polymers in controlled drug delivery , Progress in Polymer Science, 2008, Volume 33, pp. 1088-1118.
- ↑ a b Igor Galaev, Bo Mattiasson, Smart Polymers for Bioseparation and Bioprocessing , CRC Press, 2001, ISBN 978-0-415-26798-4 .
- ↑ C. Wu, X. Wang, Globule-to-Coil Transition of a Single Homopolymer Chain in Solution , Physical Review Letters, 1998, Volume 80, pp. 4092-4094.
- ↑ S. Vshivkov, AP Safronov, The conformational coil-globule transition of polystyrene in cyclohexane solution , Macromolecular Chemistry and Physics, 1997, Volume 198, 3015th
- ↑ a b Ronald Koningsveld, Walter H. Stockmayer, Erik Nies, Polymer Phase Diagrams , Oxford University Press, Oxford, 2001, ISBN 978-0-19-855635-0 .
- ↑ a b c V. Aseyev, H. Tenhu, FM Winnik, Non-ionic Thermoresponsive Polymers in Water , Advances Polymer Science, 2010, Volume 242, pp. 29-89.
- ↑ Jing Ping Chen, Allan S. Hoffman, Polymer-Protein Conjugates II. Affinity precipitation separation of human immunogammaglobulin by a poly (N-isopropylacrylamide) -protein A conjugate , Biomaterials, 1990, Volume 11, pp. 631-634.
- ↑ R. Dinarvand, A. D'Emanuele, The use of thermoresponsive hydrogels for on-off release of molecules , Journal of Controlled Release , 1995, Volume 36, pp. 221-227.
- ↑ a b c d Jan Seuring, Seema Agarwal, Polymers with Upper Critical Solution Temperature in Aqueous Solution , Macromolecular Rapid Communications, 2012, Volume 33, pp. 1898-1920.
- ^ A b A.R. Schultz, PJ Flory, Phase Equilibria in Polymer-Solvent Systems , Journal of the American Chemical Society, 1952, Volume 74, pp. 4760-4767.
- ^ C. Wohlfarth, Upper Critical (UCST) and Lower Critical (LCST) Solution Temperatures of Binary Polymer Solutions , Polymer Handbook, 87th ed., CRC press, 2006, chapter 13, pp. 19-34, ISBN 978-0-8493 -0487-3 .
- ↑ J. Hashizume, A. Teramoto, H. Fujita, Phase Equilibrium Study of the Ternary System Composed of Two Monodisperse Polystyrenes and Cyclohexane , Journal of Polymer Science, Polymer Physics Edition, 1981, Volume 19, pp. 1405-1422.
- ↑ A. Nakajima, F. Hamada, S. Hayashi, Unperturbed Chain Dimensions of Polyethylene in Theta Solvents , Journal of Polymer Science, Part C: Polymer Symposium, 1966, Volume 15, pp. 285-294.
- ↑ R. Koningsveld, AJ Staverman, Liquid-Liquid Phase Separation in Multicomponent Polymer Solutions. II. The Critical State , Journal Polymer Science, Polymer Physics Editions, 1968, Volume 6, pp 325-347.
- ↑ TG Fox, Properties of dilute polymer solutions III: Intrinsic viscosity / temperature relationships for conventional polymethyl methacrylate , Polymer, 1962, Volume 3, pp. 111-128.
- ↑ JMG Cowie, IJ McEwen, Lower critical solution temperatures of polypropylene solutions , Journal of Polymer Science: Polymer Physics Edition, 1974, Volume 12, pp. 441-443.
- ↑ Oliver Pfohl, Toshiaki Hino, John M. Prausnitz, Solubilities of styrene-based polymers and copolymers in common solvents , Polymer, 1995, Volume 36, pp. 2065-2073.
- ↑ JMG Cowie, I J. McEwen, Influence of microstructure on the upper and lower critical solution temperatures of poly (methyl methacrylate) solutions , Journal of the Chemical Society, Faraday Transactions 1: Physical Chemistry in Condensed Phases, 1976, Volume 72, p. 526-533.
- ↑ S. Fujishige, K. Kubota, I. Ando, Phase Transition of Aqueous Solutions of Poly (N-isopropylmethacrylamide) and Poly (N-isopropylmethacrylamide) , Journal of Physical Chemistry, 1989, Volume 93, pp 3311-3313.
- ↑ M. Heskins, JE Guillet, Solution Properties of Poly (Nisopropylacrylamide) , Journal of Macromolecular Science: Part A - Chemistry, 1968, Volume 2, pp 1441-1455.
- ↑ A. Kagemoto, Y. Baba, Kobunshi Kagaku, 1971, Volume 28, p 784.
- ↑ Y. Maeda, T. Nakamura, I. Ikeda, Hydration and Phase Behavior of Poly (N-vinylcaprolactam) and Poly (N-vinylpyrrolidone) in Water , Macromolecules, 2002, Volume 35, pp 217-222.
- ↑ HG Schild, DA Tirrell, Microcalorimetric Detection of Lower Critical Solution Temperatures in Aqueous Polymer Solutions , Journal of Physical Chemistry, 1990, Volume 94, pp 4352-4356.
- ^ GN Malcolm, JS Rowlinson , The Thermodynamic Properties of Aqueous Solutions of Polyethylene Glycol, Polypropylene Glycol and Dioxane , Transactions of the Faraday Society, 1957, Volume 53, pp 921-931.
- ^ S. Saeki, N. Kuwahara, M. Nakata, M. Kaneko, Upper and lower critical solution temperatures in poly (ethylene glycol) solutions , Polymer, 1976, Volume 17, pp 685-689.
- ^ GV Assche, B. Van Mele, T. Li, E. Nies, Adjacent UCST Phase Behavior in Aqueous Solutions of Poly (vinyl methyl ether): Detection of a Narrow Low Temperature UCST in the Lower Concentration Range , Macromolecules, 2011, Volume 44, pp 993-998.
- ↑ See also Jörn F. Lübben , Daniel Crespy, Matthijs de Geus, Manfred Heuberger, Monitoring the hygrothermal response of poly (vinyl methyl ether) submicron films using AFM , European Polymer Journal, Volume 48, 1, 2012, pp 209-216 .
- ^ R. Longenecker, T. Mu, M. Hanna, NAD Burke, HDH Stöver, Thermally Responsive 2-Hydroxyethyl Methacrylate Polymers: Soluble-Insoluble and Soluble-Insoluble-Soluble Transitions , Macromolecules, 2011, Volume 44, pp 8962-8971.
- ↑ P. Mary, DD Bendejacq, M.-P. Mareau, P. Dupuis, Reconciling Low- and High-Salt Solution Behavior of Sulfobetaine Polyzwitterions , Journal of Physical Chemistry B, 2007, Volume 111, pp 7767-7777.
- ↑ R. Buscall, T. Corner, The Phase-Separation Behavior of Aqueous Solutions of Polyacrylic Acid and its Partial Sodium Salts in the Presence of Sodium Chloride , European Polymer Journal, 1982, Volume 18, pp 967-974.
- Jump up ↑ Jan Seuring, Frank M. Bayer, Klaus Huber, Seema Agarwal, Upper Critical Solution Temperature of Poly (N-acryloyl glycinamide) in Water: A Concealed Property , Macromolecules, 2012, Volume 45, pp 374-384.
- ↑ Fangyao Liu, Jan Seuring, Seema Agarwal, Controlled Radical Polymerization of N-Acryloylglycinamide and UCST-type Phase Transition of the Polymers , Journal of Polymer Science A: Polymer Chemistry , 2012, Volume 50, pp 4920-4928.
- ↑ N. Shimada, H. Ino, K. Maie, M. Nakayama, A. Kano, A. Maruyama, Ureido-Derivatized Polymers Based on Both Poly (allylurea) and Poly (L-citrulline) Exhibit UCST-Type Phase Transition Behavior under Physiologically Relevant Conditions , Biomacromolecules, 2011, Volume 12, pp 3418-3422.
- ↑ Jan Seuring, Seema Agarwal, First Example of a Universal and Cost-Effective Approach: Polymers with Tunable Upper Critical Solution Temperature in Water and Electrolyte Solution , Macromolecules, 2012, Volume 45, pp 3910-3918.
- ↑ Karel Solc, Karel Dusek, Ronald Koningsveld, Hugo Berghmans, "Zero and Off-Zero Critical Concentrations in Solutions of Polydisperse Polymers with Very High Molar Masses , In: Collection of Czechoslovak Chemical Communications , 1995, Volume 60, pp 1661-1688.
- ↑ Fatema Afroze, Erik Nies, Hugo Berghmans, Phase transitions in the system poly (N-isopropylacrylamide) / water and swelling behavior of the corresponding networks , Journal of Molecular Structure, 2000, Volume 554, pp 55-68.

