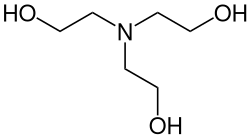
Triethanolamine
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Triethanolamine | ||||||||||||||||||
| other names | |||||||||||||||||||
| Molecular formula | C 6 H 15 NO 3 | ||||||||||||||||||
| Brief description |
viscous, hygroscopic, colorless to yellowish liquid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 149.19 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
1.13 g cm −3 |
||||||||||||||||||
| Melting point |
21 ° C |
||||||||||||||||||
| boiling point |
360 ° C |
||||||||||||||||||
| Vapor pressure |
<0.01 hPa (20 ° C) |
||||||||||||||||||
| pK s value |
7.74 |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| MAK |
DFG / Switzerland: 1 mg m −3 (measured as inhalable dust ) |
||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Triethanolamine is a colorless compound that smells slightly of fish, which quickly turns dark in the air and strongly attracts moisture and carbon dioxide .
Extraction and presentation
Triethanolamine is made by the ammonolysis of ethylene oxide . This reaction also produces 2-aminoethanol and diethanolamine .
properties
Chemical properties
Triethanolamine reacts easily with fatty acids to form triethanolamine soaps, which are not only easily soluble in water but also in mineral oils. In aqueous solution it reacts strongly basic .
use
Triethanolamine is used as a basic component in soaps and cosmetics , as a wetting agent for textiles, as a plasticizer in the leather industry, as a corrosion inhibitor or as an intermediate for the production of soaps, dispersants and water-soluble herbicides and as a grinding aid in the production of cement . In holography it is used to shift the reconstruction color. Triethanolamine is also used as a catalyst detoxifier in ammonia synthesis, since triethanolamine is strongly CO 2 attractive. It is also a pharmaceutical excipient, for example in drug gels.
Triethanolamine can also be used as a starting material in the synthesis of HN-3, a nitrogen mustard. It is a chemical weapon used in the First World War . For this reason, the substance is on List 3 of the Chemical Weapons Convention and productions of an annual quantity of 30 t or more are notifiable, and export to countries that have not signed the convention is prohibited.
To determine the pollution of the outside air with nitrogen dioxide , this is caused to react with triethanolamine in a passive collector . The nitrite resulting from the reaction is extracted and then analyzed by means of colorimetry and ion chromatography .
Health hazards / risk assessment
The vapors irritate the eyes and the respiratory tract. It has a low toxicity ( LD 50 oral rat> 5000 mg / kg, LD 50 dermal rabbit> 2000 mg / kg)
In 2012, triethanolamine was included in the EU's ongoing action plan ( CoRAP ) in accordance with Regulation (EC) No. 1907/2006 (REACH) as part of substance evaluation . The effects of the substance on human health and the environment are re-evaluated and, if necessary, follow-up measures are initiated. The reasons for the uptake of triethanolamine were concerns about consumer use , exposure of workers , high (aggregated) tonnage, other hazard- related concerns and widespread use, as well as the hazards arising from a possible assignment to the group of CMR substances and the possible hazard from sensitizing properties . The re-evaluation started in 2014 and was carried out by the United Kingdom . A final report was then published in which no changes to the existing classification were recommended.
Individual evidence
- ↑ Entry on TRIETHANOLAMINE in the CosIng database of the EU Commission, accessed on February 17, 2020.
- ↑ a b c d Entry on 2,2 ′, 2 ′ ′ - nitrilotriethanol. In: Römpp Online . Georg Thieme Verlag, accessed on May 13, 2014.
- ↑ a b c d e f g h i Entry on 2,2 ′, 2 ′ ′ - Nitrilotriethanol in the GESTIS substance database of the IFA , accessed on April 29, 2017(JavaScript required) .
- ↑ Simond, MR: Dissociation Constants of protonated amine in Water at Temperatures from 293.15 K to 343.15 K . In: Journal of Solution Chemistry . 41, 2012, p. 130. doi : 10.1007 / s10953-011-9790-3 .
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 102-71-6 or triethanolamine ), accessed on March 15, 2019.
- ↑ Chemical Weapons Conventions Implementation Act
- ↑ DIN EN 16339: 2013-11 outside air; Determination of the concentration of nitrogen dioxide using a passive collector; German version EN 16339: 2013. Beuth Verlag, Berlin, p. 7.
- ^ European Chemicals Agency (ECHA): Substance Evaluation Report and Conclusion Document .
- ↑ Community rolling action plan ( CoRAP ) of the European Chemicals Agency (ECHA): 2,2 ′, 2 ′ ′ - nitrilotriethanol , accessed on March 26, 2019.