Surfactants
Surfactants ( Latin tensus , tensioned) are substances that reduce the surface tension of a liquid or the interfacial tension between two phases and enable or support the formation of dispersions or act as solubilizers .
Surfactants mean that two liquids that are actually immiscible with one another, such as oil and water, can be finely mixed. Surfactants are also understood to mean washing-active substances ( detergents ) that are contained in laundry detergents , dishwashing detergents and shampoos . The surfactant content in detergent formulations is usually 1–40%. Modern surfactants were developed in the first half of the 20th century and have largely replaced the traditionally used surfactant soap (fatty acid salts).
When used in food technology , surfactants are known as emulsifiers .
Surfactants are also used as wetting , foaming and dispersing agents.
properties
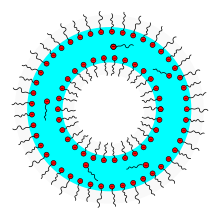
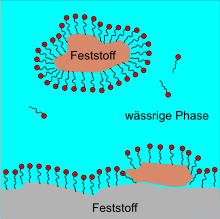
The function of surfactants can be explained by their molecular structure. Surfactants generally consist of a hydrophobic (“water-repellent”) hydrocarbon residue and a hydrophilic (“water-loving”) part of the molecule; they are said to be amphiphilic (“loving both”). In the following figures, the “water-loving” parts of the molecule are marked with the color red.
If you put surfactants in water, they first arrange themselves on the water surface. Above a critical concentration surfactants form within the water usually small, spherical aggregates , the micelles are called. The surfactant molecules align themselves in such a way that the hydrophobic ends collect inside the micelles and the hydrophilic ends are oriented in the direction of the water. With a high concentration of surfactants, rod-shaped micelles ( worm-like ) can also form. At an even higher concentration, lamellar surfactant bilayers or liposomes are formed which encapsulate water. The aggregation comes about because it is energetically more favorable. Solutions made from worm-like micelles in particular show a very complex flow behavior, which is still the subject of current research.
The surfactants form a thin layer on the surface of the water and thus lower the surface tension of the water. The surfactant molecules are also arranged here. The hydrophilic ends point in the direction of the water, the hydrophobic ends protrude in the direction of the air.
- The influence of surfactants on the surface tension can be easily demonstrated: A light object (for example a pin) is placed on a water surface (surfactant-free water). Normally this will not go under, but rather is carried by the water due to the high surface tension. If you then add small amounts of a surfactant (e.g. detergent), the surface tension is reduced. This can no longer counteract the weight that acts on the surface of the water due to the higher density of the object applied: the object sinks.
As emulsifiers, surfactants ensure that two immiscible liquids (e.g. oil in water) can mix to form an emulsion . Due to the amphiphilic character of the surfactant molecule, its fat-soluble part penetrates the oil. Due to the hydrophilic part, the oil droplet remains dispersed in the water after stirring .
We speak of wetting agents when the aim of using the surfactants is not to mix two phases, but to reduce the interfacial tension between a solid surface and a liquid. Instead of forming drops, water flows more easily from a surface. In the photo laboratory , for example, surfactants are used as wetting agents to prevent dry spots on photo materials after the final wash.
Surfactants support the detachment of small solid particles from solid surfaces, e.g. the removal of dirt particles on clothing. The solid particles are “held in suspension” in the water. Their use supports the formation and maintenance of a so-called suspension . The surfactants accumulate around the solid particles in an emulsion-like manner and prevent them from clumping together, sinking (= sedimentation ) and renewed adhesion to other solid surfaces that are themselves covered with a "surfactant layer". The solid particles coated with the surfactant form a so-called colloid with the water . As dispersing agents surfactants are referred to the solid pigments (still) liquid in a paint keep in suspension.
The formation of foam is due to the properties of surfactants. The surfactant molecules form a two-layer film with the hydrophobic ends of the surfactants forming the two surfaces. The hydrophilic ends point into the film. A strong foam development can be disturbing when using or in the presence of surfactants, which is why defoamers are used.
Anionic surfactants form insoluble precipitates with cations of the alkaline earth metals , which are generally referred to as lime soaps . Lime soaps no longer have the properties of "soluble" surfactants described above. The formation of lime soaps is due to the hardness of the water . If surfactants are used as detergents , a softener is added to the detergent .
Structure and manufacture
All surfactants are made up of a non-polar and a polar part ( functional groups ) (see polarity ). An alkyl group or an alkylbenzene group usually serves as the non-polar part . The polar part can have different structures (see table):
| Surfactants | hydrophilic group (s) |

|
|---|---|---|
| nonionic surfactants | –OH (multiple alcohols ), –O– ( ethers ) or the combination –O – CH 2 –CH 2 –OH (e.g. ethoxylates ) | |
| anionic surfactants | –COO - ( carboxylates ), –SO 3 - ( sulfonates ) or –OSO 3 - ( sulfates ) | |
| cationic surfactants | R 4 N + ( quaternary ammonium group ) | |
|
amphoteric surfactants (zwitterionic surfactants) |
mostly –COO - ( carboxylates ) and R 4 N + ( quaternary ammonium group ) |
Often attempts are made to differentiate between natural and synthetic surfactants. This distinction is not easy and does not always make sense. Naturally occurring surfactants are, for example, saponins or phospholipids such as lecithin .
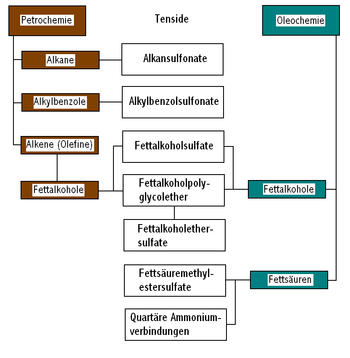
Surfactants of natural origin are, for example, soaps that are made from natural raw materials ( renewable raw materials , for example from vegetable or animal fats ) by saponification . On the basis of fats and the fatty alcohols obtained from them, surfactants such as fatty alcohol polyglycol ethers (FAE), fatty alcohol sulfates (FAS), fatty alcohol ether sulfates (FAES, sulfated fatty alcohol polyglycol ethers) and methyl ester sulfonates (MES, sulfonated fatty acid methyl esters ) can also be produced. Also from renewable raw materials one gets to sugar surfactants such. B. alkyl glycosides from hexoses or pentoses and fatty alcohols. However, its manufacture requires a profound chemical reaction .
Synthetic surfactants are made from petroleum raw materials ( petrochemicals ) or derived products synthesized from them , such as alkanes , benzene , alkylbenzenes , olefins , ethylene oxide and fatty alcohols , for example into alkylbenzenesulfonates (ABS), secondary alkanesulfonates (SAS) and corresponding surfactants such as based on Fats derived fatty alcohols converted.
An important property of a surfactant is its biodegradability . The Washing and Cleaning Agent Act (WRMG) requires every surfactant manufacturer to be able to biodegrade at least 90% (often 95%) of the surfactant. Tests to determine the biological (BOD) and chemical oxygen demand (COD) are often carried out for this purpose. The type of biodegradation of some surfactants (e.g. alkylbenzenesulfonate ) is known very precisely. When alkylphenol polyglycol ethers were suspected of being toxic to fish, the manufacturers immediately withdrew the product from the market.
history
Soap-like compounds were used as early as 2500 BC. Reported in Sumerian cuneiform documents . By boiling olive oil with wood ash ( potash ), soap-like compounds could be obtained. Surfactant-like products made from fats were also known to the Egyptians, Greeks, Romans, Germans and Gauls.
Soap-like products were also made from wood ash and fats in the Middle Ages and the Renaissance . It was only through the synthetic soda production from table salt , sulfuric acid and lime using the Leblanc process that soap could be produced cheaply.
In the 20th century, detergents were also increasingly needed for cleaning textiles in washing machines .
Normann K. Adam developed a readily available surfactant, tetrapropylene benzene sulfonate (TPS). At the beginning of the 1960s, this surfactant covered 65% of the surfactant demand in the western world . However, due to the poor biodegradability, piles of foam formed in rivers. From 1964, more biodegradable, linear alkylbenzenesulfonates (LAS) were developed.
Since the beginning of the 1980s, research has also concentrated on the search for renewable surfactant raw materials. Henkel has been producing alkyl polyglycosides since 1990 . These contain a sugar residue as a hydrophilic part of the molecule and are therefore classified as sugar surfactants . Since the sugar residue has no charge, they are classed as nonionic surfactants.
The surfactants used today meet the legally required degree of primary degradation , although there are considerable differences in the level of degradation ultimately achieved . The degree of primary degradation relates to the loss of interfacial activity . However, the final degradation is only complete when the organic compound has been completely converted. This final dismantling is not covered by laws.
use
food industry
Certain surfactants are used as emulsifiers or foaming agents in food. The approved food additives are listed in the article List of approved food additives in the European Union .
The alkalization or saponification of cocoa fat in drinking cocoa powder serves to reduce the surface tension of the milk and to achieve faster wetting or suspension of the semi-fat cocoa powder.
Detergents and detergents
Surfactants are used in laundry detergents , dishwashing detergents , shampoos , shower gels , etc. to increase the “solubility” of fat and dirt particles in water that adhere to the laundry or to the body.
The surfactants used include linear alkylbenzenesulfonates (LAS), alkyl polyglycosides (APG), esterquats (EQ), fatty alcohol ethoxylates (FAEO), fatty alcohol sulfates (FAS) and fatty alcohol ether sulfates (FES). However, alkylphenol polyglycol ethers , alkylphenol ethoxylates (APEO) and tetrapropylene benzene sulfonate (TPS) are no longer used .
Fabric softener consists of cationic surfactants that prevent laundry from becoming dry when dry.
Pharmacy and Cosmetics
Emulsifiers are a prerequisite for producing water-in-oil emulsions and the like. a. for making skin creams . Furthermore, they are necessary for a large number of suspensions in order to obtain medicinal substances in liquid form.
Pesticides
Plant protection products contain surfactants for better wetting ( spreading ) of the plant. The most common wetting agent is ethoxylated tallow amine . Trisiloxanes or polyoxyethylated fatty alcohols are also used.
biochemistry
In biochemistry surfactants are inter alia to denaturation of proteins and the solubilization of membrane proteins used:
- Sodium Lauryl Sulphate (SLS or SDS)
- Cetyltrimethylammonium bromide (CTAB)
- Octoxinol 9
- Polysorbate 20
- Polyalkylene glycol ether research
- Salts of bile acids such as cholate or deoxycholate
- Digitonin
- Dodecyl maltoside
- Octyl glucoside
- Cleavable surfactants
earth sciences
In geology and palaeontology, surfactants are often used to gently prepare sediment samples. This method can be used, for example, to prevent the formation of sulphurous acid in samples containing pyrite , which would be the case if they were processed with the hydrogen peroxide otherwise used for sample preparation .
technology
Plastic test
Surfactants have a special application in plastics technology . Aqueous surfactant solutions are used here to test the susceptibility of polymer materials to stress cracking . Surfactants are also used to shorten the failure time of long-term tests; This is especially used in crack growth tests on polyethylene . Wetting agents are used in the full notch creep test for testing polyethylene pipelines .
Antistatic agents
Ionic surfactants also act as external antistatic agents to prevent electrostatic charging of plastic surfaces ( ESD protection ). Both anionic and cationic surfactants are used for this.
Textile finishing
The use of perfluorinated surfactants , e.g. B. Fluorotelomer alcohols (FTOH), as coating materials for textiles, carpets and building products, imparts or improves the water and grease repellent properties of these products. As representatives of the PFC group , however, they are under criticism because they are persistent and practically do not break down naturally.
Cooling lubricant
Surfactants are used in water- mixed cooling lubricants ( water-in-oil emulsions ) to cool and lubricate during metal cutting.
photography
This prevents drying spots and streaks during film development . A wetting agent is added to the last water bath (final wash).
Printer ink
Surfactants control the consistency of the ink in inkjet printers . Too few surfactants lead to clumping of the color pigments , too many make the ink too fluid when printing.
Paper recycling
Surfactants help in paper recycling to detach the printing ink particles from the paper fibers and in transporting the printing ink to the surface during deinking (printing ink removal ).
Fire fighting
One method of fire fighting is to extinguish with "relaxed water ", also known as "net water" , that is, water with a greatly reduced surface tension. On the one hand, this has the advantage that the extinguishing water can penetrate better into burning materials such as wood or fabric and thus has an even better cooling effect. On the other hand, because of its effect as a flow improver, extinguishing water mixed with surface-active substances can be sprayed over a greater distance with the same pumping capacity. However, the latter effect is not used consciously. Special foaming agents ( AFFF ) for fighting liquid fires contain perfluorinated surfactants , which form a gas-tight liquid film between the material to be burned and the foam, which at the same time gives the foam carpet better sliding properties and thus enables larger liquid fires to be extinguished in the first place.
medicine
In medicine, wetting agents are used as an additive in ointments and in secretolysis ( e.g. Tyloxapol , Polysorbate 80 ). They reduce the surface tension of liquids and dissolve dried secretions from the epithelial layer through intensive wetting of the mucus balls and plugs. However, the assessment of their therapeutic value is inconsistent.
Natural occurrence
Certain caterpillars of insects spit on predators with a surfactant-containing secretion . This has a deterrent effect on the attacking ants and enables the caterpillars to escape. This behavior has been observed in caterpillars from Southeast Asia originating moth beet armyworm .
Economic importance
The world production of surfactants in 2000 was 10.5 million tons. Anionic surfactants (56% of the world production of surfactants) and nonionic surfactants (35% of the world production of surfactants) are the economically most important classes of surfactants. In 2010, around 6.5 million tons of anionic surfactants were in demand worldwide. Together with the nonionic surfactants, these two groups account for approx. 85% of the global surfactant demand.
In 2008, about 3.0 million tons of surfactants were produced in Western Europe, 1.6 million tons in the USA, 1.4 million tons in China and 0.98 million tons in Japan. In 2008, 1.22 million tons of anionic surfactants, 1.41 million nonionic surfactants, 0.28 million tons of cationic surfactants and 80,000 tons of amphoteric surfactants were produced in Western Europe.
The most important surfactant in the world is linear alkyl benzene sulfonate (LAS) with an annual production of 4 million tons. Another important surfactant of steadily growing importance (current production volume: 90,000 tons) is methyl ester sulfonate (MES, sodium alpha-sulfoalkanecarboxylic acid methyl ester). Annual production is estimated at 1 million tons by 2020. “Green surfactants” such as N- acyl glutamate, N- acyl sarcosinate and sorbitan acid esters are particularly important in the cosmetics industry because of their higher price.
A very large international manufacturer of surfactants (especially for LAS) is the Sasol company . Other important companies in the surfactant market are BASF , Clariant , Cognis , Huntsman, Shell , in Russia Nizhnekamskneftekhim ( Russian : Нижнекамскнефтехим) and Kirishinefteorgsintez (Russian: КиришинефФтеоргсинтез) in China; India Indian Petrochemicals Corp. Ltd. A further growing surfactant market is expected in the Asian region in particular.
Individual evidence
- ↑ Beck, R .: Physical properties of anionic surfactant systems with divalent counterions and their mixtures with zwitterionic surfactants and cosurfactants. 2004, accessed November 20, 2019 .
- ↑ strey.pc.uni-koeln.de: Rheology: Viscoelastic behavior of worm-like micellar surfactant solutions ( Memento of the original from December 31, 2017 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ a b Bernd Fabry: Surfactants, properties, raw materials, production, applications . In: Chemistry in Our Time . tape 25 , no. 4 , 1991, pp. 214–222 , doi : 10.1002 / ciuz.19910250407 .
- ↑ Umweltlexikon-online.de: Tenside , accessed on May 27, 2013.
- ↑ Syngenta: Application technology for agriculture - Additives - Important additives (p. 11)
- ↑ Calbiochem Booklet: Detergents (PDF; 619 kB).
- ↑ http://www.solvay.com/en/binaries/EMEA-Catafor%20Formulation-BROCHURE_2015%20-%20Copie-270934.pdf Product information from Solvay Novecare (USA), accessed on July 24, 2017.
- ↑ Franz v. Bruchhausen, G. Dannhardt, Siegfried Ebel, August-Wilhelm Frahm, Eberhard Hackenthal, Ulrike Holzgrabe: Hager's handbook of pharmaceutical practice . Springer-Verlag, 2013, ISBN 978-3-642-57880-9 , pp. 292 ( limited preview in Google Book search).
- ↑ a b F.H. Meyers, E. Jawetz, A. Goldfien: Textbook of Pharmacology For students of medicine of all study stages and for doctors . Springer-Verlag, 2013, ISBN 978-3-642-66183-9 , p. 350 ( limited preview in Google Book Search).
- ^ F. Kauffmann: Negotiations of the German Society for Internal Medicine, sixty-second congress . Springer-Verlag, 2013, ISBN 978-3-662-40971-8 , pp. 88 ( limited preview in Google Book search).
- ↑ Surfactants market study by Ceresana Research, February 2012.
- ↑ HG Hauthal: Tenside, Sustainability: Raw materials, products, processes, SÖFW-Journal, 6-2008, p. 10.
- ↑ HG Hauthal: Tenside, Sustainability: Raw materials, products, processes, SÖFW-Journal, 6-2008, p. 11
- ^ Karl Winnacker, Leopold Küchler, Roland Dittmeyer: Nutrition, Health, Consumer Goods . In: Technical Chemistry . tape 8 . Wiley-VCH, 2005, ISBN 3-527-30773-7 , pp. 795 ff .
literature
- Bernd Fabry: Surfactants - properties, raw materials, production, applications. In: Chemistry in Our Time . 25 (4), pp. 214-222 (1991), doi: 10.1002 / ciuz.19910250407
- Fredric M. Menger, Jason S. Keiper: Gemini Surfactants. In: Angewandte Chemie . 112, pp. 1980-1996 (2000), doi : 10.1002 / 1521-3757 (20000602) 112: 11 <1980 :: AID-ANGE1980> 3.0.CO; 2-D
- Günter Wagner: detergents . Scientific series. Chemistry and ecology, Ernst Klett Verlag Stuttgart 1993, ISBN 3-12-993663-7
- Tilo Kaiser, Winfried Schwarz, Matthias Frost: Discharge of substances in soils - an assessment of the hazard potential of platinum group elements, lanthanides, organotin compounds, phthalates, nonylphenol, surfactants, polycarboxylic acids, cleaning and disinfecting agents, veterinary drugs and feed additives . Logos-Verl., Berlin 1998, ISBN 978-3-89722-089-8 .
Web links
- Calbiochem Booklet: A Guide To The Properties And Uses Of Detergents , 2001 (PDF; 597 kB)
- Learning aids on detergents and surfactants