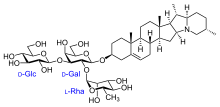
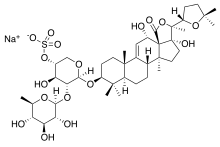
Saponins

R 2 = different alkyl residues
Saponins ( Latin sapo 'soap' ) are glycosides of steroids , steroid alkaloids (nitrogen-containing steroids) or triterpenes . One speaks therefore of steroid saponins, steroid alkaloid saponins and triterpene saponins (although it should be noted that the classification of “steroid alkaloid saponins” as saponins is controversial and in case of doubt the terminology “steroidal glycoalkaloids” is to be preferred). Due to the large number of possible carbohydrate structures and the great structural variability of the aglycones , this group of substances has a correspondingly large structural diversity and thus a large variability in biological properties.
The term sapon glycoside is a pleonasm and is therefore incorrect.
history
Soaps as such have been known since ancient times. However, it was only Leopold Gmelin who defined the term “saponins”.
Johann Friedrich Zittmann made from the roots of various Smilax TYPES a decoction ago, the 1795 by Johann Christian Theden is called "decoction Zittmanni" and against syphilis is used.
Tennet began using the Senega root as an expectorant in 1736 . In 1896 W. von Schulz was able to isolate the contained saponin senegin. During the world wars, the drug became very rare and expensive, so that it was replaced by Radix Saponariae albae or primrose roots and flowers , which are still used today as expectorants.
properties
Saponins are so named because they often make a soap-like foam when shaken with water . Accordingly, a general mode of action of this class of compounds is also in the foreground, which is related to the detergent property . Saponins generally form stable foams, often show hemolytic activity , influence membrane permeability, complex cholesterol , and sometimes have a bitter taste (exceptions: glycyrrhizin , which makes licorice taste sweet , and the very sweet mogrosides from the Chinese plant Siraitia grosvenori ) and are piscizid (toxic to fish).
Occurrence
Saponins are widespread in higher plants, especially in nutrient-rich tissues such as roots, tubers, leaves, flowers, and seeds. Saponins have been described in over 90 plant families. In a study of 1730 Central Asian plant species, saponins were found in 76% of families. Saponins can be found in vegetables such as soybeans, chickpeas, peanuts, mung beans, broad beans, lentils, peas, spinach, oats, eggplant, asparagus, fennel, garlic, sugar beet, tomatoes, green peppers, potatoes ( solanine ), onions, cassava and yams . They are also part of tea, ginseng or jiaogulan . Saponins are secondary plant substances .
The juice from the rhizomes of the real soap herb was used as a detergent early on, hence the botanical genus name Saponaria . Saponins occur in high concentrations in chestnuts and in the bark of the South American soap bark tree ( Quillaja saponaria ), the latter also known as Panama bark . For ecological reasons, Indian soap nuts , whose washing effect is due to the high concentration of saponins, have recently aroused broader interest. Furthermore, saponins are found in marine microorganisms and sea rollers ( holothurins ). Various other plants contain saponins, e.g. B. cyclamen ( Cyclamen spp. ), Barringtonia asiatica , bittersweet nightshade , auriculatum Cestrum , ivy , Guajacum , Gypsophila struthium , Jiaogulan ( Gynostemma pentaphyllum ), corn cockle ( Agrostemma githago ) Lungenkraut , alfalfa , Polygala senega , primrose , Quinoa ( Chenopodium quinoa ), soap root , dog carnation , chickweed ( Stellaria media ), washing walnut and ginseng .
Chemical structures
The structural classification of the saponins is initially based on the aglycones, the so-called sapogenins, since the saccharide part itself varies considerably in one and the same natural source and several different glycosylation patterns can usually be found next to one another. In the case of sapogenins, on the other hand, one usually finds a much more limited structural diversity in one source. In both cases, the variability is mostly due to related metabolic pathways and their intermediate steps, so that a whole series of similar compounds with partly similar properties but graded in their biological effect can be found.
Monodesmosid saponins have one sugar chain, bisdesmosid saponins have two sugar chains, which are bound at different positions on the aglycone.
- Steroid sapogenins with a C-27 basic structure:
- Spirostanes, in which the side chain forms a bicyclic spiroketal (E and F ring) fused to the D ring (e.g. digitogenin )
- Furostans, in which part of the side chain forms a furan ring (E-ring) fused to the D-ring
- rearranged C-27 skeletons compared to cholestane
- Steroid alkaloid sapogenins with a C-27 basic structure and nitrogen atom mostly in the side chain (here, too, there are variants similar to those of steroid sapogenins)
- Triterpene sapogenins with a C-30 basic structure and a .:
- Cycloartane: Tetracyclic cholestane backbone and an additional fused-on cyclopropane ring
- Dammarane, Tirucallane and Cucurbitane: three fused six-membered rings (A – C) and one five-membered ring (D) fused to the C-ring
- Holothurinogenins, which are derived from the holostane ((20S) -20-hydroxy-5α-lanostane-18-acid lactone) or lanostane . The group has three fused six-membered rings (A – C) and one double five-membered ring (D, E) fused to the C-ring, the outer one of which is a lactone ; this ring also has a non- fused tetrahydrofuran ring. The glycosides form the group of holothurins .
- Lupane (fabric group) : four fused six-rings (A – D) and a five-ring fused to the D-ring (E)
- Oleanane (see structural formula of balloon flower saponins), Ursane and Taraxasterane: five fused six-membered rings (A – E). Often the methyl group C-28 in these saponins is oxidized to acid, e.g. B. in saponins, which are derived from the aglycon gypsogenin .
Biological function
Saponins probably serve the plants as defensive substances, for example against fungal attack and insect damage. Since plants do not have an active immune system like vertebrates, harmful organisms are often fought chemically. During the ripening of nightshade plants such as tomatoes and potatoes , the poisonous steroid alkaloid saponins are converted into non- toxic steroid saponins by denitrification (enzymatic removal of nitrogen).
biosynthesis
Saponins come from the phytosterol anabolism . About the cytosolic mevalonate pathway formed DMAPP and IPP , forming the basis for the formation of 2,3-oxido squalene , which marks the last common precursor molecule to a crossroads of phytosterols, steroid saponins and Steroidalkaloidsaponinen on one side and triterpene saponins on the other side. So-called oxidosqualene cyclases (also: triterpene synthases) catalyze cyclization cascades of oxidosqualene which result in the formation of cycloartenol in the case of the former or different triterpenes such as dammaran , lupeol and β-amyrin in the further biosynthesis of the latter. It is not known at which step steroid saponins or steroid alkaloid saponins split off from further phytosterol biosynthesis.
The formation of the initial sapogenin framework is often followed by the modification of individual positions. In most cases, the first instance is the introduction of hydroxy , keto or carboxy groups . The majority of these oxidations are probably catalyzed by " cytochrome P450 " enzymes. Hydroxy and carboxy groups can then serve as a point of attack for linking with side groups of the most varied of chemical origins, such as, for example, smaller aliphatic and aromatic acids or even sugar residues.
The glycosylation of the aglycone, i.e. the linkage with sugar residues, is usually necessary to ensure the biological activity of saponins. Typical saponin sugar side chains consist of 2–5 monosaccharide units and are linked to the C3 hydroxyl group and / or (if present) C28 carboxy group of the sapogenin via ether or ester bonds . The most common sugar residues found in sapon glycosylation patterns are glucosyl , galactosyl , glucuronyl , rhamnosyl , xylosyl and arabinosyl units. So far identified enzymes involved in the synthesis of saponin sugar side chains all belong to the class of family 1 UDP - glycosyltransferases and each catalyze the linkage of a single monosaccharide residue with the sapogenin or the growing saponin sugar side chain.
Saponins in phytotherapy (herbal medicine)
The saponins as a subgroup of the glycosides occupy an important place among the therapeutically effective components of medicinal plants . In accordance with their great structural diversity, a large number of different biological-pharmaceutical properties are also observed. It will u. a. tonic, anti-inflammatory, diuretic, expectorant / expectorant and hormone-stimulating properties were observed. They also support the absorption of other ingredients from the intestine and, on the other hand, bind cholesterol . It is also believed to have a preventive effect against colon cancer through an inhibitory effect on cell division in the intestine.
However, saponins must not get into the bloodstream, as many of them have a hemolytic (blood-dissolving) property even in small quantities, i.e. they lead to the destruction of the red blood cells . The hemolytic property is used as a standard quantitative method in blood testing. In the case of inflammation of the intestinal wall, saponins can increase the permeability of the intestinal wall.
The main suppliers of saponins are legumes , but they are also found in beetroot , asparagus and sugar beet as well as in various medicinal plants such as the daisy , the immortal herb and the horse chestnut .
analysis
The analysis of saponins is usually carried out by LC-MS , HPLC , gas chromatography , sometimes also by thin layer chromatography . The presence of saponins can be checked by shaking it, which forms a foam that stands for more than 10 minutes. The bark of Entada phaseoloides serves as a positive control .
Applications
Saponins are effective components of the adjuvant QS-21 . They strengthen the cellular immune response .
literature
- DE Fenwick, D. Oakenfull: Saponin content of food plants and some prepared foods. In: Journal of the science of food and agriculture. Volume 34, Number 2, February 1983, pp. 186-191, PMID 6855202 .
- Lawrence A. Johnson: Soybeans. Elsevier, 2015, ISBN 978-0-12-804352-3 , p. 312.
Web links
Individual evidence
- ^ A b Wolf-Dieter Müller-Jahncke , Christoph Friedrich , Ulrich Meyer: Medicinal history . 2., revised. and exp. Ed. Wiss. Verl.-Ges, Stuttgart 2005, ISBN 978-3-8047-2113-5 , pp. 183 .
- ^ Wolf-Dieter Müller-Jahncke , Christoph Friedrich , Ulrich Meyer: Medicinal history . 2., revised. and exp. Ed. Wiss. Verl.-Ges, Stuttgart 2005, ISBN 978-3-8047-2113-5 , pp. 184 .
- ↑ a b c d e K. Hostettmann: Saponins. Cambridge University Press, 2005, ISBN 978-0-521-02017-6 . P. 18.
- ↑ Entry on Holothurine. In: Römpp Online . Georg Thieme Verlag, accessed on April 26, 2012.
- ↑ K. Papadopoulou, RE Melton, M. Leggett, MJ Daniels, AE Osbourn: Compromised disease resistance in saponin-deficient plants. In: Proceedings of the National Academy of Sciences . 96, 1999, pp. 12923-12928, doi: 10.1073 / pnas.96.22.12923 .
- ↑ Tetsuro Shinoda, Tsuneatsu Nagao, Masayoshi Nakayama, Hiroaki Serizawa, Masaji Koshioka, Hikaru Okabe, Akira Kawai: Identification of a Triterpenoid Saponin from a Crucifer, Barbarea vulgaris, as a Feeding Deterrent to the Diamondback Moth, Plutella xylostella In: Journal of Chemical Ecology. 28, 3, pp. 587-599, doi: 10.1023 / A: 1014500330510 .
- ↑ Jörg M. Augustin, Vera Kuzina, Sven B. Andersen, Søren Bak: Molecular activities, biosynthesis and evolution of triterpenoid saponins. In: Phytochemistry , Vol. 72, Issue 6, April 2011, pp. 435-457, doi: 10.1016 / j.phytochem.2011.01.015 .
- ↑ HX Sun, Y. Xie, YP Ye: Advances in saponin-based adjuvants. In: Vaccine. Volume 27, Number 12, March 2009, pp. 1787-1796, doi: 10.1016 / j.vaccine.2009.01.091 , PMID 19208455 .