Triterpenes
Triterpenes are natural substances that are made up of three terpene units, i.e. a total of six isoprene units with a total of 30 carbon atoms . In individual cases, the number of carbon atoms in oxidized representatives can differ, examples of which are (+) - azadirone or limonine .
Classification of the triterpenes
Grouping according to the number of rings
Most compounds that are assigned to the triterpenes have 30 carbon atoms. Nevertheless, there are over 4000 compounds that are classified in this group of substances. They are basically differentiated in the number of cycles within the compound.
| Classification of the triterpenes | |||||
|---|---|---|---|---|---|
| Number of rings |
Common name of a representative |
Structural formula | |||
| 0 | Squalene |
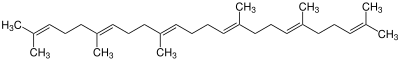
|
|||
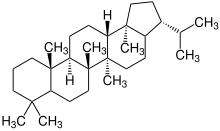
| 4th | Lanosterol |

|
|||
| 5 | Betulin |

|
|||
Triterpenes that do not have a ring system are called linear triterpenes; if they have 4 rings, the compounds are assigned to the tetracyclic triterpenes and with 5 rings analogously to the pentacyclic triterpenes.
Assignment of selected representatives
Individual triterpenes are usually assigned even more precisely than just the number of rings in the molecule . Very similar structures can be found in many representatives of this group of substances. We are often talking about the basic structure or the skeleton of the molecules. In the case of triterpenes, three basic structures in particular appear frequently. These are the Steran - which Baccharan and - Hopan scaffold.
Linear triterpenes
The simplest triterpenes are squalane and squalene. They serve as the backbone of the tetracyclic and pentacyclic triterpenes, which are mainly produced by biosynthesis . A characteristic step is the so-called folding of the squalene. This creates the various basic structures, such as the gonan, baccharan or hopan skeleton. A total of over 200 different scaffolds are already known. Starting from these scaffolds, however, many different products can arise through further reaction steps.
With respect to the isoprene rule , the triterpenes also offer some irregularities. Simple connections often meet the isoprene rule. Examples are squalane and squalene. Compounds whose biosynthesis consist of many different reaction steps often do not meet the original isoprene rule, such as lanosterol. These cannot be completely broken down into isoprene units.
Tetracyclic triterpenes
The tetracyclic triterpenes are built up from the sterane framework (outdated: gonane framework). The compounds within this group are also known as steroids . However, there are also steroids that are not classified as triterpenes. An example of this is cholesterol, which is often found in animal fats. Some important representatives of the tetracyclic triterpenes are listed in the following table:
| Subgroups of the tetracyclic triterpenes | |||||
|---|---|---|---|---|---|
| Representative | Structural formula with a sterane framework marked in
green |
Subgroup | Area of application Occurrence |
||
| (+) - Dammar-24-en-3 β -20S-diol |
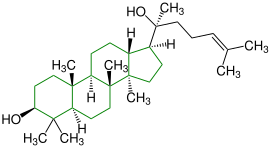
|
Dammarane |
 In plaster and as a binding agent in paints |
||
| (+) - azadirone |
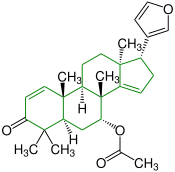
|
Apotirucallane |
 insecticides |
||
| (-) - Tirucalla-7,24-dien-3 β -ol |
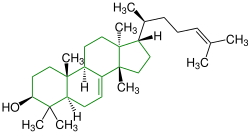
|
Tirucallane |
 Black tea |
||
| Lanosterol |
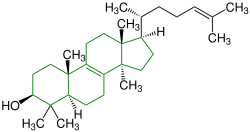
|
Lanostane |
 Fungal metabolites, components of a fir tree, component of yeast (picture) |
||
| Cimicifugenol |
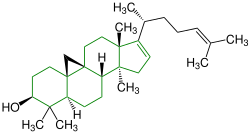
|
Cycloartanes | Substitute for estrogen | ||
| Cucurbitacin F |

|
Cucurbitane |
 pharmaceutical use (inhibits the growth of tumor cells ) |
||
Pentacyclic triterpenes
In the pentacyclic triterpenes, the compounds are based on two essential basic structures, the baccharane framework and the hopane framework. Some industrially relevant representatives of the Baccharan type are listed in the following table:
| Subgroups of the pentacyclic triterpenes |
|||||
|---|---|---|---|---|---|
| Representative | Structural formula with baccharan framework marked
green |
Subgroup | Area of application Occurrence |
||
| (+) - 1,11-dihydroxy-20 (29) -lupen-3-one |
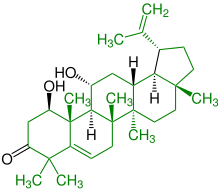
|
Lupanes |
 Antibiotic , lowering blood cholesterol, part of sage plants |
||
| Betulin |

|
Lupanes |
 Treatment against AIDS |
||
| (+) - Soyasapogenol C. |

|
Oleananes |
 Foaming agent in shampoos |
||
| (+) - Ursolic acid |

|
Ursane |
 Treatment of leukemia , emulsifier in food; occurs in apple and pear peels |
||
In addition to the baccharan skeleton, some pentacyclic triterpenes also have a hopan skeleton. The representatives, however, are not of great industrial relevance, but they do occur in some plant species.
Individual evidence
- ↑ Eberhard Breitmeier: Terpene: Aromen, Fragrances, Pharmaka, Pheromone 2nd edition, Wiley-VCH, Weinheim, 2005, ISBN 978-3-527-62369-3 , pp. 87-93.
- ^ A b c Eberhard Breitmeier: Terpenes: Aromen, Fragrances, Pharmaka, Pheromone 2nd edition, Wiley-VCH, Weinheim, 2005, ISBN 978-3-527-62369-3 , pp. 85-86.
- ^ Ran Xu, Gia C. Fazion, Seiichi PT Matsuda: On the origins of triterpenoid skeletat diversity. In: Phytochemistry . , No. 65, 2004, pp. 261-291, doi: 10.1016 / j.phytochem . 2003.11.014 .
- ↑ Leopold Ruzika: The role of fragrances in my chemical life's work , Helv. Chim. Acta 54: 1753-1759 (1971).
- ↑ a b Eberhard Breitmeier: Terpenes: Aromen, Düfte, Pharmaka, Pheromone 2nd edition, Wiley-VCH, Weinheim, 2005, ISBN 978-3-527-62369-3 , pp. 94-100.