Holothurines
| Holothurines | ||
| Surname | Holothurine A | Holothurine B |
| Structural formula |  |
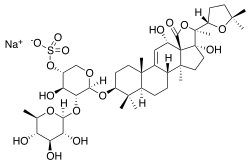
|
| other names | ||
| CAS number | 38-26-6 | 11052-32-7 |
| PubChem | 44559168 | 23674754 |
| Molecular formula | C 54 H 85 NaO 27 S | C 41 H 63 NaO 17 S |
| Molar mass | 1221.29 g mol −1 | 882.98 g mol −1 |
| Physical state | firmly | |
| Melting point | 239-240 ° C | 252 ° C |
| LD 50 | 9 mg kg −1 (mouse, iv ) | 14 mg kg −1 (mouse, intraperitoneal ) |
The holothurins are a group of saponins ( triterpene saponins), which can be found as toxic ingredients in sea rollers (holothuroidea). These are stored by the animals in Cuvier's tubes , which can be actively sprayed when threatened. Previously known representatives of this group of substances are holothurin A and holothurin B, occasionally echinoside A (CAS number: 75410-53-6) is also referred to as holothurin A2.
The starfish , which are related to sea cucumbers , form similar substances, the asterosaponins .
Structure and properties
Holothurins are glycosides ; the basic aglycone bodies ( holothurinogenins ) are derived from the holostane ((20 S ) -20-hydroxy-5 α -lanostane-18-acid lactone) or lanostane . All holothurins are sulfated on the sugar part of the glycoside .
The holothurins are slightly psychoactive , but there are no known uses of the entheogenic effects. However, it was found that holothurins have an inhibitory effect on the growth rate of various tumor cells in mice , which makes them interesting for cancer medicine .
safety instructions
If they come into contact with the skin, holothurines can cause severe, burning pain and, if they come into contact with the eyes, cause irritation and even blindness. When absorbed systemically, the toxins can lead to symptoms of paralysis, muscle spasms and complaints in the digestive system, and in larger quantities, death from respiratory paralysis.
Since sea cucumbers are considered a delicacy in many Asian countries , the Cuvier's tubes containing the toxins must be removed before preparation or consumption.
In mice, both holothurin A had a LD 50 value of 9 mg / kg iv -Gabe, and holothurin B with an intraperitoneal value of 14 mg / kg strongly toxic.
literature
- Chanley, JD; Ledeen, R .; Wax, J .; Nigrelli, RF; Sobotka, Harry: Holothurine. I. insulation properties, and sugar components of holothurin A . In: Journal of the American Chemical Society . 81, No. 19, 1959, pp. 5180-5183. doi : 10.1021 / ja01528a040 .
- I Kitagawa: Structure of holothurin A a biologically active triterpene-oligoglycoside from the sea cucumber holothuria leucospilota brandt . In: Tetrahedron Letters . 20, No. 16, 1979, p. 1419. doi : 10.1016 / S0040-4039 (01) 86166-9 .
Individual evidence
- ↑ Shu-Yu Zhang, Yang-Hua Yi, Hai-Feng Tang: Bioactive Triterpene Glycosides from the Sea Cucumber . In: Journal of Natural Products . tape 69 , no. 10 , October 2006, p. 1492-1495 , doi : 10.1021 / np060106t .
- ↑ Parvataneni Radhika, Vallurupalli Anjaneyulu, Potluri Venkata Subba Rao, TN Makarieva, AI Kalinovosky: Chemical examination of the Echinoderms of the Indian Ocean: The triterpene glycosides of the sea cucumbers: Holothuria nobilis, Bohadschia aff. tenuissina and Actinopyga mauritana from Lakshadweep, Andaman and Nicobar Islands . In: Indian Journal of Chemistry Sect. B: Organic Chemistry including Medicinal Chemistry . 41B, no. 6 , 2002, pp. 1276-1282 .
- ↑ a b Entry on Holothurin A in the ChemIDplus database of the United States National Library of Medicine (NLM)
- ↑ a b Biochemical Pharmacology. Vol. 16, p. 1617, 1967.
- ↑ a b Entry on Holothurin B in the ChemIDplus database of the United States National Library of Medicine (NLM)
- ↑ a b Miyazaki Daigaku Nogakubu Kenkyu Hokoku . Bulletin of the Faculty of Agriculture, University of Miyazaki. Vol. 26, p. 97, 1979.
- ↑ a b c d e Entry on Holothurine. In: Römpp Online . Georg Thieme Verlag, accessed on April 26, 2012.
- ^ Atta-ur-Rahman: Studies in Natural Products Chemistry . Elsevier, 2008 ( full text in Google Book Search).
- ^ Wissenschaft-Online-Lexika: Entry on "Holothurine" in the Lexikon der Biochemie, accessed on April 26, 2012.
- ↑ Tomofumi Miyamoto, Kenichi Togawa, Ryuichi Higuchi, Tetsuya Komori: Constituents of holothuroidea, I. Isolation and structures of three triterpenoid aglycones, cucumechinol A, B, and C, from the sea cucumber Cucumaria echinata . In: Liebig's annals of chemistry . tape 1990 , no. 1 , January 22, 1990, p. 39-42 , doi : 10.1002 / jlac.199019900106 .