Cycloartenol
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
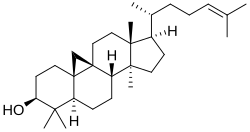
|
||||||||||
| General | ||||||||||
| Surname | Cycloartenol | |||||||||
| other names |
|
|||||||||
| Molecular formula | C 30 H 50 O | |||||||||
| Brief description |
white solid |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 426.72 g mol −1 | |||||||||
| Physical state |
firmly |
|||||||||
| Melting point |
|
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Cycloartenol is an organic chemical compound , more precisely a tetracyclic triterpene alcohol with an additional cyclopropane ring.
Occurrence
Cycloartenol occurs naturally in many plants and has been found, for example, in the milky sap of the milkweed family (Euphorbiaceae), in the potato ( Solanum tuberosum ) and the nugget ( Strychnos nux vomica ). It is present, at least in traces, in all green, photosynthesis- active plants, since it is the first detectable cyclization product of 2,3-epoxysqualene in the course of the plant biosynthesis of sterols or phytosterols . The biosynthesis takes place from squalene via 2,3-epoxysqualene. In animals, the function of the compound is taken over by lanosterol .
properties
Cycloartenol is a white solid.
Individual evidence
- ↑ a b c d data sheet Cycloartenol, ≥90% (GC) from Sigma-Aldrich , accessed on April 24, 2014 ( PDF ).
- ↑ a b c Entry on Cycloartenol. In: Römpp Online . Georg Thieme Verlag, accessed on April 24, 2014.
- ↑ Lexicon of Biochemistry: Cycloartenol - Lexicon of Biochemistry , accessed on April 24, 2014.
- ↑ U. Eppenberger, L. Hirth, G. Ourisson: Anerobic cyclization of squalene-2,3-epoxide to cycloartenol in tissue cultures of Nicotiana tabacum L. In: European Journal of Biochemistry. 8, 1969, pp. 180-183, doi : 10.1111 / j.1432-1033.1969.tb00512.x .
- ↑ Gerhard Richter: Metabolic physiology of plants: Physiology and biochemistry of the primary and secondary metabolism . Georg Thieme, 1997, ISBN 3-13-152346-8 , p. 347 ff . ( limited preview in Google Book search).