Critical micelle concentration
In the chemistry describes the critical micelle concentration , or CMC ( English critical micelle concentration ), the concentration of a surfactant , from which micelles form. If the concentration is increased further, almost all of the additional molecules are converted into micelles.
The CMC is a characteristic property of surfactants such as those used in detergents, soaps, etc.
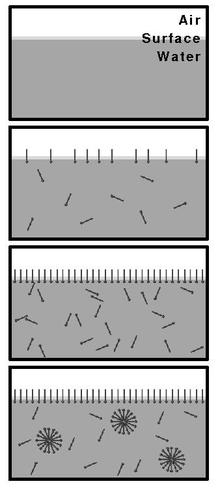
Behavior of surfactants at different concentrations
With a concentration of surface-active substances (such as surfactants) below the CMC, the molecules move freely and individually in the liquid (as a monomer ) and preferably position themselves with their hydrophobic part of the molecule on the free surface. At concentrations below the CMC, the surface tension of the liquid decreases as the concentration of the surfactants increases. When the CMC is reached, the surfactant molecules begin to form micelles. If the concentration is increased further, the number of micelles formed increases. Since the available "positions" at the interfaces of the liquid are exhausted at the latest when the CMC is reached, the surfactants now have no further influence on the free surface of the liquid and the surface tension remains - in an ideal system - constant above the CMC.
The CMC depends on
- the ionic strength and the structure of the head group of the surfactant as well as the length and shape of the lipophilic hydrocarbon chain,
- the dispersant (usually water) and its pressure and temperature,
- the presence of other surface-active substances or electrolytes ,
- the amount of interfaces present.
The CMC decreases with increasing ionic strength . Micelles only form above the temperature range of the Krafft point .
With a compact volume, the extent of the existing interfaces has only a minor influence on the CMC. If, however, the expansion of the interfaces in the medium is greatly increased by blowing in finely divided air bubbles, then surfactant molecules accumulate at the interfaces, foam is formed and the number of micelles is reduced, until finally no more micelles remain when the CMC is undershot.
Notes for different systems
The CMC is defined as the critical concentration inside the liquid , i.e. without its interfaces on the surface and the vessel walls. This note is of no relevance for most systems, especially when the number of surfactant molecules in the liquid itself (in the “bulk phase”, in the “bulk” or in the “volume phase”) compared to the number of surfactant molecules in the surface is great. Thus, for these cases, the concentration of the surfactants can easily be determined via the amount of surfactants added and the volume of liquid (or mass).
In some special cases, for example when the free surface is large compared to the volume of the liquid, for example due to air bubbles, the surfactant concentration in the liquid can no longer be determined using the method described. So bind z. B. in bubble columns large amounts of surfactants to the rising bubbles, so that the local concentration in the liquid can fall below the CMC. This method can also be used to remove surfactants from a solution ( flotation ). Similar considerations can be made for emulsions and suspensions .
Web links
- Interface Chemistry Institute for Physical and Theoretical Chemistry, TU Braunschweig
- Theory on the Krüss website
literature
- SA Baeurle, J. Kroener: Modeling effective interactions of micellar aggregates of ionic surfactants with the Gauss-Core potential. In: J Math Chem. 36, 2004, pp. 409-421.
Individual evidence
- ^ Compendium of Chemical Terminology IUPAC
- ^ Interface chemistry PCI, TU Braunschweig
- ↑ JA Reynolds, Charles Tanford : Binding of dodecyl sulfate to proteins at high binding ratios. Possible implications for the state of proteins in biological membranes. In: Proceedings of the National Academy of Sciences . Volume 66, Number 3, July 1970, pp. 1002-1007, PMID 5269225 , PMC 283150 (free full text).