Tyloxapol
| Structural formula | |||||||||
|---|---|---|---|---|---|---|---|---|---|
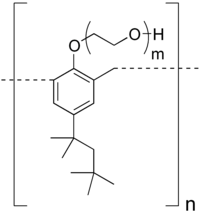
|
|||||||||
| General | |||||||||
| Surname | Tyloxapol | ||||||||
| other names |
|
||||||||
| CAS number | 25301-02-4 | ||||||||
| Monomers / partial structures |
|
||||||||
| Qualitative molecular formula |
(C 15 H 21 O (C 2 H 4 O) m ) n |
||||||||
| Molar mass estimation |
variable |
||||||||
| PubChem | 71388 | ||||||||
| ATC code | |||||||||
| DrugBank | DB06439 | ||||||||
| Brief description |
viscous amber liquid |
||||||||
| Drug information | |||||||||
| Drug class |
Expectorans |
||||||||
| Mechanism of action |
Wetting agent, surfactant |
||||||||
| properties | |||||||||
| density |
1.10 g cm −3 |
||||||||
| solubility |
soluble |
||||||||
| safety instructions | |||||||||
|
|||||||||
| Toxicological data | |||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||
Tyloxapol is a non-ionic, liquid polymer that is primarily used as a wetting agent in expectorant preparations ( mucolytics ) and as an inhalative drug carrier. Because of these properties, Tyloxapol has been used to dissolve mucus in diseases of the respiratory tract and lungs for more than 50 years. Tyloxapol is also found in some eye medicines (ophthalmics).
chemistry
Tyloxapol is a polymeric compound made up of the synthetic building blocks octylphenol , formaldehyde and ethylene oxide . First, 4-tert-octylphenol is condensed with formaldehyde (analogous to phenolic resins ) and then ethylene oxide is added ( ethoxylation ). The resulting macromolecule is a nonionic homeopolar compound, sterilizable and neutral to acids, bases and salts. Tyloxapol has a density of 1.10 grams per cubic centimeter, and a flash point of> 113 ° C .
Substance properties
Tyloxapol is a nonionic, is readily soluble in water wetting agent surfactant and surfactants are surface-active substances. (Engl surface active agent. Surfactant which the interfacial tension of liquids (synonym:) surface tension ) may decrease. This enables them to wet surfaces and allow immiscible liquids to flow into one another. These properties are based on their chemical structure consisting of a hydrophobic (water-repellent) and a hydrophilic (water-loving) part of the molecule. In contrast to anionic or cationic surface-active substances, Tyloxapol is a non-ionic, homeopolar compound, which is of particular importance for its medical use (compatibility with mucous membranes).
use
Tyloxapol solutions are used as an inhalative wetting agent (surfactant) to improve the liquefaction and removal of bronchial secretions. As a surface-active substance, Tyloxapol has a purely physical effect by reducing the surface tension of airway mucus and helping to liquefy the sputum . This liquefaction of bronchial mucus (mucolysis) facilitates the expulsion of mucus z. B. coughing up, which is a therapeutic goal of all respiratory diseases associated with mucus. In addition, Tyloxapol solutions serve as a carrier for inhaled drugs (e.g. antibiotics , corticoids , antiallergics ) and can help to improve their distribution and effect in the bronchial tract. The currently (as of December 2015) only finished preparation with the main active ingredient Tyloxapol sold in Germany is Tacholiquin®, (manufacturer Bene Arzneimittel, Germany ). B. in bronchitis , bronchiolitis , sinus inflammation , pneumonia , bronchiectasis , asthma or pseudo croup , preferably as inhalation using a compressed air nebulizer is used. Tacholiquin® had been on the market in Germany since September 1, 1956 as a mucolytic drug. Since the active ingredient Tyloxapol has a purely physical effect, i.e. has no main pharmacological , metabolic or immunological effect, Tacholiquin 1% solution was classified as a class 2a medical product in 2007.
Clinical information
application areas
In the case of diseases of the respiratory tract and the lungs, which are characterized by highly viscous sputum , Tyloxapol solution helps to liquefy the viscous mucus and to loosen the secretions so that their removal is facilitated. Accordingly, the main areas of application are in the treatment of acute and chronic irritation of the airway mucous membranes, such as those present in sinus, pharyngeal and trachitis, bronchitis and bronchiolitis (inflammation of the large and small bronchi), whooping cough , bronchial asthma (chronic inflammatory disease of the airways, associated with attack-like shortness of breath), pseudo croup (viral inflammation of the larynx, associated with cough and shortness of breath). A large number of published reports on Tyloxapol applications are available for a number of other indications (see section Application examples from practice ). Many clinical and laboratory studies on Tyloxapol date from the 1950s to 1970s. In previous studies, Tyloxapol was also called Triton WR 1339. Alevaire is a previous brand name for a tyloxapol solution with a lower concentration (0.125% tyloxapol) than tacholiquin (1%) but the same excipients ( sodium hydrogen carbonate and glycerin ).
Method of application and dosage
Tyloxapol solution can be used as inhalation and instillate ( tracheal cannula - intratracheal, bronchoscope - intrapulmonary ). For inhalation , nebulization using an aerosol device is required. Commercially available inhalation devices are suitable, in particular compressed air, vibrating membrane or ultrasonic nebulizers . Evaporation with hot water is unsuitable. Tyloxapol can be inhaled diluted with saline solutions ( brine ) or sterile or distilled water. As an aerosol carrier, it can also be mixed with inhaled medications, such as germ-inhibiting substances (e.g. bacitracin , tyrothricin , erythromycin , nystatin ), bronchodilator agents (e.g. orciprenaline , salbutamol , theophylline ), corticoids, anesthetics or antiallergics . Because of the wetting properties of Tyloxapol, better distribution and deposition of inhaled medication can be achieved, especially into the lower airways and into poorly ventilated regions of the bronchial tree. In severe cases and in threatening conditions of respiratory insufficiency , continuous inhalation via the ventilator can be used in intensive care.
For instillation by trained specialists, Tyloxapol is diluted 1: 1 with distilled water or saline solution and applied locally to the mucous membrane using a pipette , syringe or bronchoscope, e.g. B. before suctioning off the mucus in tracheal cannula wearers or in bronchoalveolar lavage .
Contraindications (contraindications) and restrictions on use
Tyloxapol should not be used if you are known to be hypersensitive to any component of the preparation, if you have pulmonary edema or if fluid has accumulated in the lungs. Particular caution is required in the event of a build-up of secretion due to impaired removal of mucus from the airways, e.g. B. in the (rare) malignant ciliary syndrome , since mobilized mucus here requires a willingness to suction. For use in pregnancy and lactation No data are available; in these cases, drugs or medical devices should generally only be used after consulting a doctor. There are no age restrictions with regard to the use of Tyloxapol in children, since there is extensive experience with the use of Tyloxapol in children of all ages, including premature babies (see section Examples of use from practice ). Tyloxapol solutions must not be administered intravenously.
Side effects and tolerability
When inhaling Tyloxapol, the first deep breaths can trigger an urge to cough, which disappears after the mucous membrane has been wetted. Hypersensitivity to the solution occurs very rarely. Nausea occasionally occurred in allergy sufferers. Otherwise, Tyloxapol has proven to be very well tolerated even with long-term use. A review article on 15 years of clinical use of Tyloxapol comes to the conclusion that the inhaled substance is well tolerated and safe. In various studies, patients were continuously treated with Tyloxapol for up to four years without any specific side effects being observed. Both children and adults tolerated up to 1,500 ml tyloxapol per day as continuous aerosol administration without irritation or damage to the airway mucosa. Miller administered tyloxapol to premature babies weighing less than 1,000 g without experiencing any skin or other side effects to the extent that he believed tyloxapol was contraindicated in premature babies. Silverman was unable to determine any benefit or harm from nebulizing Tyloxapol in the incubator in 200 premature babies.
Tyloxapol is not absorbed by the mucous membrane to a clinically relevant extent . There are no known interactions of Tyloxapol with medicinal products.
pharmacology
Physical mechanisms of action
The active ingredient Tyloxapol influences the respiratory system through different mechanisms of action of a purely physical nature:
- Reduction of surface tension
- Reduction of viscosity ( viscosity ) of secretions ( secretolysis ) and slime ( mucolysis )
- Loosening of deposits by reducing adhesion (improved secretomotor function)
This results in indirect funding
- coughing up secretions and mucus
- the disease-related impaired ciliary activity ( mucociliary clearance )
- the mucosal contact of inhaled concomitant drugs.
The longer Tyloxapol acts on the mucus and the higher its concentration, the thinner the sputum becomes. This liquefaction of bronchial mucus (mucolysis) together with the promotion of the secretomotor system facilitate the expulsion of mucus z. B. by coughing up.
Experimental Findings
It can be shown experimentally that tyloxapol reduces the viscosity of the sputum even in low concentrations, i.e. it tends to liquefy it. Ravenel (1953) found a 10-20% decrease in viscosity. Wilde (1973) started dilution series and observed the greatest decrease in sputum viscosity when 5% tyloxapol solution was added. He sees the mechanisms of this effect as the reduction of the surface tension of the bronchial secretion and the penetration of the active substance into the mucus. As a result, deposits adhering to the mucous membrane are more easily detached, the cilia of the mucous membrane are released again and can transport the mucus away (secretomotor effect).
toxicology
The acute oral LD50 of tyloxapol in the mouse is 1000 mg / kg and in the rat 5000 mg / kg body weight. The LD50 in rabbits after intravenous administration is 300 mg / kg body weight. When used by inhalation, Tyloxapol, as a non-ionic wetting agent, has a favorable toxicological profile compared to anion or cation-active substances. Repeated nebulization (1 hour daily up to 4 weeks) of guinea pigs with concentrations above 50% did not result in any changes in the airway tissues. In white rats, high inhalation doses (8 hours daily on 6 consecutive days) did not lead to pathological changes in the lungs or the behavior of the animals.
Application examples from practice
Tyloxapol has been administered as an inhalation drug to millions of patients worldwide since the 1950s. However, the early studies predominantly had the character of observational studies and, due to the time, often did not meet today's requirements for clinical studies with regard to randomization, double blinding, comparison groups, etc. In addition to the indications mentioned in the section Application areas , Tyloxapol was used in the following areas:
Pulmonary medicine
Documented applications are bronchial asthma, abscesses (accumulation of pus in a tissue cavity), atelectasis (non-ventilated lung area as a result of collapsed lung sections), chronic obstructive bronchitis, bronchiectasis (expansion of the bronchi), pneumonia, emphysema, tracheitis (trachitis), inflammation of the trachea.
Ear, nose and throat medicine
Applications in sinus infections, rhinitis sicca, etc. are documented. a. Uses of tyloxapol for respiratory complications in premature and newborn babies and the promotion of infectious sputum.
surgery
The use after operations in the chest and abdominal cavity to prevent and / or eliminate a cessation of excretion of secretions in the event of mechanical respiratory impairment was described.
Trade names
Alevaire (USA), Blink N Clean, Cationorm, Enuclene, Exosurf neonatal, Tacholiquin (EU), Talof
Individual evidence
- ↑ a b c d e f Datasheet Tyloxapol at Acros, accessed on December 27, 2015.
- ↑ a b c Entry on Tyloxapol in the DrugBank of the University of Alberta , accessed December 27, 2015.
- ↑ a b c d Stieve FE wetting agents in aerosol therapy, journal. F. Aerosol Research and Therapy No. 3/1957
- ↑ a b Wilde: Surface tension and viscosity measurements on sputa under the influence of Tacholiquin, notabene medici, 8th year, issue 1, 1978
- ↑ a b c d Wilde: Aerosol therapy with expectorants in the pulmonary practice, notabene medici, 5th year, no. 2, 26-33, 1975
- ↑ Tainter, Nachod, Bird: Alevaire as a mucolytic agent, New Engl. J. Med. 253: 764-767, 1955
- ↑ a b c d Csernohorszky et al. The use of tacholiquin in the prevention and treatment of postoperative pulmonary complications. Zentralblatt für Chirurgie, 88; 1963: 435-438
- ↑ a b Khanal A et al. Surfactant Driven Post-Deposition Spreading of Aerosols on Complex Aqueous Subphases. 1: High Deposition Flux Representative of Aerosol Delivery to Large Airways. J Aerosol Med Pulm Drug Deliv 2015; 5: 382-393
- ↑ a b c Red List 2015
- ↑ EC Directive 93/42 / EEC for medical products
- ^ Fireplace W drug, therapy criticism 2011 / episode 1 Hans Marseille Verlag GmbH Munich
- ↑ Tacholiquin® 1% instructions for use, Bene-Arzneimittel GmbH, Munich
- ↑ Marcinkowski AL et al. Postdeposition dispersion of aerosol medications using surfactant carriers. J Aerosol Med Pulm Drug Deliv. 2008 Dec; 21 (4): 361-70. doi : 10.1089 / jamp.2008.0699
- ↑ Sharma R et al. Surfactant Driven Post-Deposition Spreading of Aerosols on Complex Aqueous Subphases. 2: Low Deposition Flux Representative of Aerosol Delivery to Small Airways. J Aerosol Med Pulm Drug Deliv 2015; 5: 394-405
- ^ Miller JB Detergent aerosol therapy: a 15-year review of laboratory and clinical tolerance, Clinical medicine, 1967; 74: 37-40
- ↑ Miller, Conyers, Dinhoffer: A simple, safe bronchographic technique for children, J. Pediat. 1950, 36: 721ff
- ↑ Sadove MS et al. Postoperative aerosol therapy, JAMA 1954; 156: 759ff.
- ↑ Miller: Wetting agents in antibiotic mists, JAMA 1955; 159: 738ff
- ^ Silverman, Andersen: Controlled Trial of Effects of Alevaire Mist on Premature Infants. JAMA 1970, 157: 1093-1096
- ^ Ravenel: New techniques of humidification in pediatrics, JAMA, Vol. 151: 707-711, 1953
- ↑ Miller, Boyer: A nontoxic detergent for aerosol use in dissolving viscid bronchopulmonary secretions, J. Pediatr., 767-771, 1952