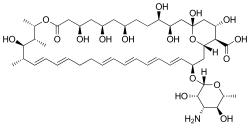
Nystatin
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Nystatin | |||||||||||||||||||||
| other names |
3- (4-Amino-3,5-dihydroxy-6-methyl-tetrahydropyran-2-yl) oxy-19,25,27,29,32,33,35,37-octahydroxy-18,20,21-trimethyl -23-oxo-22,39-dioxabicyclo [33.3.1] nonatriaconta-4,6,8,10,14,16-hexaen-38-carboxylic acid ( IUPAC ) |
|||||||||||||||||||||
| Molecular formula | C 47 H 75 NO 17 | |||||||||||||||||||||
| Brief description |
yellowish solid |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| Mechanism of action |
Pore formation in the cell membrane of fungi due to the accumulation of ergosterol during the synthesis of the cell membrane |
|||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 926.09 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
250 ° C (decomposition from 160 ° C) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Nystatin is a polyene - macrolactone from Streptomyces noursei , a actinobacterium the genus of Streptomycetes . It is a fungistatic and is used as an antifungal to treat fungal infections (such as Candida albicans and Aspergillus fumigatus ).
history
Nystatin was the first antifungal agent isolated from Streptomyces noursei in 1948 by Elizabeth Lee Hazen and Rachel Fuller Brown of the New York State Department of Health. The Streptomyces strain from which they isolated nystatin was from the garden soil of friends with the name Nourse and was thus called noursei . Hazen and Brown named NYSTA tin in 1954 after the N ew Y ork Sta te Department of Health.
effect
Nystatin attaches to ergosterol in the cell membrane of fungi and thus impairs the integrity of the cell membrane. Pores arise in the cell wall through which potassium ions (K + ) can escape from the inside of the cells ( ionophoric effect) and thus lead to cell death of the fungus. So far, no development of resistance in Candida fungi to nystatin has been proven.
application
Nystatin is not absorbed in the intestine and can therefore only act locally in the digestive tract when administered orally. Possible side effects include diarrhea, nausea, and vomiting.
A main area of application is the therapy of vaginal mycoses .
Nystatin is often used prophylactically to prevent systemic mycoses in patients at increased risk of fungal infections, such as patients with AIDS and low CD4 + T helper cell counts and patients undergoing chemotherapy , and for prophylaxis against recurrence in chronic recurrent vaginal mycoses. It is also used in the administration of antibiotics on neonatal wards, because antibiotic therapy often results in a selection advantage for fungal pathogens.
In veterinary medicine, nystatin is used to treat intestinal mycoses .
Trade names
Adiclair (D), Biofanal (D), Candio-Hermal (D, A), Lederlind (D), Moronal (D), Multilind Suspension (CH), Mycostatin (A, CH), Mykundex (D), Nystaderm, numerous Generics (D, CH)
Candio-Hermal plus (D), Multilind Heilsalbe (D, CH), Mycolog (CH), Mykoderm (D), Mykundex Heilsalbe (D), Nystalocal (CH), Topsym polyvalent (CH)
Web links
- Nystatin Topical Powder (PDF; 275 kB)
- Nystatin application
literature
- Vicente, MF et al. (2003): Microbial natural products as a source of antifungals . In: Clin. Microbiol. Infect. Vol. 9, pp. 15-32. PMID 12691539 doi : 10.1046 / j.1469-0691.2003.00489.x (currently unavailable)
- Marianne Abele-Horn: Antimicrobial Therapy. Decision support for the treatment and prophylaxis of infectious diseases. With the collaboration of Werner Heinz, Hartwig Klinker, Johann Schurz and August Stich, 2nd, revised and expanded edition. Peter Wiehl, Marburg 2009, ISBN 978-3-927219-14-4 , pp. 256 and 258.
Individual evidence
- ↑ a b c data sheet nystatin from Sigma-Aldrich , accessed on May 29, 2011 ( PDF ).
- ↑ Entry on nystatin. In: Römpp Online . Georg Thieme Verlag, accessed on May 30, 2014.
- ^ Digging for a Cure. Antibiotics in Action. ( Memento from June 15, 2010 in the Internet Archive )
- ^ The antifungal drug nystatin. Inventor of the Week. .
- ^ Namesake: New York State Institute .