Tetrapropylene benzene sulfonate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
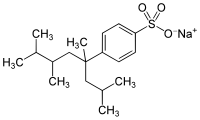
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Tetrapropylene benzene sulfonate | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 18 H 29 NaO 3 S | |||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 348.47 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Tetrapropylene benzene sulfonate (TPS) is a chemical compound from the class of sulfonates and an alkyl benzene sulfonate that is effective as an anionic surfactant . Since the tetrapropylene sulfonate, which is highly branched in the alkyl side chain, only biodegrades to around 20-30%, it was replaced by linear alkylbenzenesulfonates (LAS) such as dodecylbenzenesulfonate as early as the 1960s . The foaming of the rivers by TPS led to the first detergent and cleaning agent law in 1961.
Tetrapropylene benzene sulfonate is obtained from 1-dodecene .
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ a b Entry on Tetrapropylenebenzyl sulfonate in the ChemIDplus database of the United States National Library of Medicine (NLM)
- ↑ Entry on tetrapropylene benzene sulfonate. In: Römpp Online . Georg Thieme Verlag, accessed on January 21, 2014.
- ↑ Henkel: Primary degradation of surfactants and corresponding test methods .