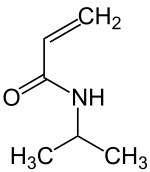
N -isopropyl acrylamide
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | N -isopropyl acrylamide | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 6 H 11 NO | |||||||||||||||
| Brief description |
white to pale yellow crystalline solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 113.16 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point | ||||||||||||||||
| boiling point | ||||||||||||||||
| Vapor pressure |
3 hPa at 83 ° C |
|||||||||||||||
| solubility |
soluble in water, methanol and isopropyl acetate |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
N -isopropylacrylamide is an N -substituted acrylamide whose nitrogen atombearsan isopropyl group . The substance has met withgreat interestas a monomer for thermoresponsive polymers or temperature- and pH-sensitive hydrogels forming poly ( N -isopropylacrylamide) .
Manufacturing
Due to the expensive reactants, the reaction of acryloyl chloride with excess isopropylamine is more suitable for synthesis in the laboratory ,
can be achieved with a product yield of over 80%.
The reaction of acrylamide with excess 2-bromopropane also leads to unsatisfactory yields of NIPAM of approx. 66%.
In a mixture of concentrated sulfuric acid with acetic acid , propene reacts with acrylonitrile at 80 ° C to form N -isopropyl acrylamide.
The same patent describes the usual industrial production route of N -isopropyl acrylamide in a Ritter reaction of acrylonitrile with isopropanol in the presence of concentrated sulfuric acid.
Depending on the reaction temperature and duration, yields of 90% to "almost quantitative" are achieved.
A variant of this reaction with an acidic zeolite catalyst to generate the carbenium ion from isopropanol supplies NIPAM in 93 percent yield.
To suppress the polymerization in solution, the reaction mixtures are often inhibitors, such as. B. phenothiazine or 4-methoxyphenol (MEHQ) added.
properties
During the synthesis, N -isopropyl acrylamide is obtained as a white to cream-colored crystalline solid, which tends to polymerize when stored at higher temperatures and is therefore often mixed with polymerisation inhibitors.
Applications
The homopolymerization of N -isopropyl acrylamide, e.g. B. with AIBN as a radical initiator, the water-soluble poly (N-isopropylacrylamide), the solvated linear polymer chains with increasing temperature above the so-called provides lower critical temperature mixture ( English lower critical solution temperature , LCST) collapse into a dense tangle.
In the presence of the crosslinker N , N ′ -methylene bisacrylamide, water-swellable and thermosensitive smart hydrogels can be produced by radical polymerization of N -isopropylacrylamide , which shrink dramatically above the so-called volume phase transition temperature (VPTT) of 33 ° C. The LCST of linear polymers is close to the VPTT of cross-linked hydrogels; H. the hydrogel behaves almost like a concentrated polymer solution.
By copolymerization with z. B Acrylic acid and crosslinking are accessible to both thermo- and pH-sensitive hydrogels.
Applications of these smart hydrogels based on N -isopropylacrylamide copolymers include: a. in the controlled drug release .
Individual evidence
- ↑ a b c d e f g Data sheet N-Isopropylacrylamide from Sigma-Aldrich , accessed on November 25, 2015 ( PDF ).
- ↑ a b c d e Entry on N-Isopropylacrylamide (stabilized with MEHQ) at TCI Europe, accessed on November 25, 2015.
- ↑ a b c Patent US2719176 : Continuous method of making N-substituted amines. Filed on Feb. 5, 1953 , published on Sept. 27, 1955 , applicant: Eastman Kodak Co., Inventor: HW Coover, Jr., NH Shearer, Jr
- ↑ a b G. Panambur, I. Koltover, p batch Elle, Designing temperature and pH sensitive NIPAM based polymer
- ↑ Patent CN101239927 : Applied on February 7, 2007 , published March 21, 2012 , Applicant: 重庆 融 海 超声 医学 工程 研究 中心 有限公司, Inventor: 叶方伟, 王智彪, 田 耘 博 (English title: Method for preparing monomer of temperature -sensitive polyisopropyl acrylamides ).
- ↑ Patent US4835312 : Production process of N-Substituted amide compounds. Applied July 2, 1986 , published May 30, 1989 , Applicants: 501 Mitsui Chemicals, Inc., Inventors: H. Itoh, T. Nakagawa, A. Nitta.
- ↑ X. Chen, H. Matsuda, T. Okuhara: Efficient catalytic synthesis of N-isopropyl acrylamide from acrylonitrile and isopropanol . In: Chem. Lett. tape 8 , 1999, p. 799-800 , doi : 10.1016 / S0926-860X (00) 00629-3 .
- ^ Y. Okada, F. Tanaka: Cooperative Hydration, Chain Collapse, and Flat LCST Behavior in Aqueous Poly (N-isopropylacrylamide) Solutions . In: Macromolecules . tape 38 , no. 10 , 2005, pp. 4465-4471 , doi : 10.1021 / ma0502497 .
- ↑ M. Constantin, M. Cristea, P. Ascenzi, G. Fundueanu: Lower critical solution temperature versus volume phase transition temperature in thermoresponsive drug delivery systems . In: eXPRESS Polym. Lett. tape 5 , no. 10 , 2011, p. 839-848 , doi : 10.3144 / expresspolymlett.2011.83 .
- ↑ F. Eeckman, AJ Moes, K. Amighi: Poly (N-Isopropylacrylamide) copolymers for constant temperature controlled drug delivery . In: Int. J. Pharm. Volume 273 , no. 1–2 , 2004, pp. 109-119 , doi : 10.1016 / ijpharm.2003.12.013 .





