Knight reaction
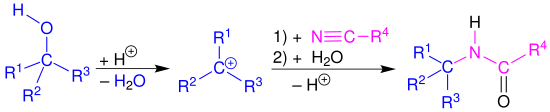
The Ritter reaction is a name reaction of organic chemistry and named after the American chemist John Joseph Ritter. It is used for the synthesis of N -alkylamides from nitriles . This requires substrates that can form carbenium ions (e.g. various alkylating agents in the presence of strong mineral acids ). Primary , secondary and tertiary alcohols as well as benzyl alcohol and tert -butyl acetate and isobutylene react very well as alkylating agents.
It has recently become possible to carry out the Ritter reaction with cycloalkanes in the presence of a strong oxidizing agent, a copper catalyst and a Lewis acid with high yields of carboxamides .
Reaction mechanism
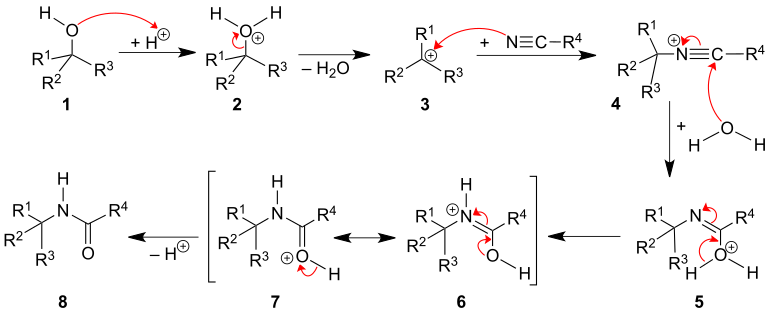
The Ritter reaction is described in this section using the example of a tertiary alcohol 1 . The first reaction step is initiated by protonation of the hydroxyl group in 1 in order to obtain a carbenium ion 3 as an alkylating agent after elimination of water . This is followed by a nucleophilic attack by the nitrile. The resulting nitrile ion 4 is hydrolyzed with water . After a proton rearrangement ( 5 → 6 ) and the subsequent cleavage of a proton, the desired amide 8 is obtained.
Individual evidence
- ↑ Ritter, JJ, Minieri, PP; J. Am. Chem. Soc. , 1948 , 70 , 4045.
- ↑ Ritter, JJ, Kalish, J .; J. Am. Chem. Soc. , 1948 , 70 , 4048.
- ↑ Krimen, LI, Cota, DJ; Org. React. 1969 , 17 , 213-325 (review).
- ↑ Lebedev, MY, Erman, MB; Tetrahedron Lett. 2002 , 43 , 1397-1399; doi : 10.1016 / S0040-4039 (02) 00057-6 .
- ^ Bishop, R .; In Comp. Org. Synth. ; Trost, BM, Fleming, I .; Eds .; Pergamon Press: New York, 1992; Vol. 6, 261-300 (review article).
- ↑ Ritter, JJ, Kalish, J .: α, α-Dimethyl-β-Phenethylamine In: Organic Syntheses . 44, 1964, p. 44, doi : 10.15227 / orgsyn.044.0044 ; Coll. Vol. 5, 1973, p. 471 ( PDF ).
- ↑ Parris, CL: N-Benzylacrylamide In: Organic Syntheses . 42, 1962, p. 16, doi : 10.15227 / orgsyn.042.0016 ; Coll. Vol. 5, 1973, p. 73 ( PDF ).
- ↑ Fernholz, H., Schmidt, HJ; Angew. Chem. , Int. Ed. Closely. , 1969 , 8 , 521; doi : 10.1002 / anie.196905211 .
- ↑ Sven Doye : An intermolecular Ritter reaction on alkanes . In: Chemistry in Our Time . tape 46 , no. 3 , 2012, p. 137 , doi : 10.1002 / ciuz.201290033 .
- ^ Z. Wang: Comprehensive Organic Name Reactions and Reagents Volume 3, Wiley Verlag, 2009, p. 2399, ISBN 978-0-471-70450-8 , (3-volume set).