Ammonium phosphate
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
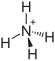 
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Ammonium phosphate | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | (NH 4 ) 3 PO 4 | ||||||||||||||||||
| Brief description |
white powder with a pungent ammonia-like odor |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 149.12 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.61 g cm −3 |
||||||||||||||||||
| solubility |
good in water (580 g l −1 at 20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Ammonium phosphate (more precisely triammonium phosphate ) is an ammonium salt of phosphoric acid . It forms readily water-soluble crystals as a trihydrate.
In addition to triammonium phosphate, there are also mono-ammonium phosphate (NH 4 ) H 2 PO 4 , di-ammonium phosphate (NH 4 ) 2 HPO 4 and ammonium polyphosphates [NH 4 PO 3 ] n .
presentation
Ammonium phosphate can be produced by reacting diammonium hydrogen phosphate with gaseous ammonia .
It is also possible to display it by neutralizing ammonia with phosphoric acid :
properties
Ammonium phosphate is unstable in the solid state. It breaks down with the release of ammonia . The trihydrate forms crystals with the space group P 2 1 / c (space group no. 14) .
use
Ammonium phosphate and ammonium polyphosphates are used as flame retardants and fire protection coatings.
Individual evidence
- ↑ a b c data sheet Triammonium Phosphate at hmlindia.com (PDF; 209 kB), accessed on October 13, 2019.
- ^ A b A. F. Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 101st edition. Walter de Gruyter, Berlin 1995, ISBN 3-11-012641-9 .
- ↑ a b Serox: ammonium phosphate
- ↑ D. Mootz, H. Wunderlich: The crystal structure of (NH 4 ) 3 PO 4 .3H 2 O, triammonium orthophosphate trihydrate. In: Acta Crystallographica Section B Structural Crystallography and Crystal Chemistry. 26, pp. 1826-1835, doi : 10.1107 / S056774087000496X .
- ↑ Agency for renewable raw materials: Market overview - insulating materials made from renewable raw materials , see table on p. 29; accessed in September 2016.

