Claude-Louis Berthollet
Claude Louis, comte Berthollet (born December 9, 1748 in Talloires in the Duchy of Savoy , † November 6, 1822 in Arcueil ), was a French chemist and doctor .
Life
Claude-Louis Berthollet came from a middle-class family in the Savoy . His father Louis Berthollet was a notary by profession and married to Philiberte Donier. Berthollet was the sixth of nine children, of whom only he and his younger sister reached adulthood. He began to study medicine in Annecy on Lake Annecy , but after a short time he moved to the University of Turin , where he received his doctorate in medicine in 1768 after a four-year stay with a royal scholarship . First he spent another four years in Piedmont, before settling in Paris as a doctor and researcher in 1772, where he deepened his knowledge of chemistry .
Here he studied chemistry with Pierre-Joseph Macquer in the Jardin du Roi , and with Jean-Baptiste-Michel Bucquet at the medical faculty of the University of Paris, faculté de médecine . Berthollet was influenced by Antoine Laurent de Lavoisier and worked with Gaspard Monge . In collaboration with Louis Bernard Guyton de Morveau , Antoine Laurent de Lavoisier and Antoine François de Fourcroy , the Méthode de Nomenclature Chimique was created in 1787 .
In 1779 he married Marguerite-Marie Baur (* 1760), who originally came from Piedmont and had been a French citizen since 1778. Their only son, Amedée Barthélemy Berthollet, was born in 1780 and died in 1810. His son's suicide was a personal tragedy for him.
He was temporarily the personal physician of Duke Louis Philippe I de Bourbon, duc d'Orléans (1725–1785) and, due to his excellent reputation as a chemist, became a member of the French Academy of Sciences in 1780 . In 1784 he took on a job as director of a manufacture for the manufacture of tapestries ( Gobelins ), manufacture royale des Gobelins . In 1791 he published his findings on dyes in the Éléments de l'art de la teinture .
Berthollet develops a process for the production of gunpowder from potassium chlorate (KClO 3 ) as a substitute for saltpetre . After all, there was a lack of natural saltpetre in France during the French Revolution and the subsequent Napoleonic Wars . In 1786, together with the mathematician and physicist Gaspard Monge (1746-1818) and the mathematician and chemist Alexandre-Théophile Vandermonde (1735-1796) , he improved the methods of increasing the quality of steel production. His work impressed Napoleon Bonaparte (1769-1821), who recruited him in 1798 for his Egyptian expedition from 1798 to 1801, where he co-founded the Institut d'Egypte on site .
On his return to Paris he became the leading chemist at the Institut de France and was able to set up a private laboratory on the outskirts of Paris at his country estate in Arcueil. In 1806 he was elected a foreign member of the Göttingen Academy of Sciences . He had a close relationship with Napoleon Bonaparte. So he helped z. B. in 1806 with a substantial loan to settle the debts of the Berthollets, especially caused by Mrs. Marguerite-Marie Berthollet. In 1804 he was appointed senator and officer of the honor guard , Garde d'Honneur , by Napoleon Bonaparte ; at the same time he received the title of Count (Comte). In his capacity as senator, however, he voted in 1814 for the impeachment of Napoléon Bonaparte. In the course of the Restoration of the Bourbons , he received a seat as a peer of France .
Berthollet spent his old age in Arcueil near Paris, where his well-equipped laboratory attracted many high-ranking researchers. This led to the Société d'Arcueil , a discussion and research group that also included Alexander von Humboldt (1769–1859), Jean-Baptiste Biot (1774–1862), Pierre-Simon Laplace (1749–1827) and Louis Jacques Thénard ( 1777–1857) belonged. The progress of the circle was published in 3 volumes between 1807 and 1817 under the title Memoires de la Société d'Arcueil . From 1789 he was a Fellow of the Royal Society . In 1820 he was elected an Honorary Fellow of the Royal Society of Edinburgh . In 1822 Berthollet was elected to the American Academy of Arts and Sciences . Berthollet died on November 6, 1822, of an anthrax infection, ulcère charbonneux as the recipient of many honors and awards in Arceuil.

Scientific achievements
He researched dyes and bleaching agents ( chlorine and hypochlorite ) and determined the chemical composition of ammonia (NH 3 ), hydrocyanic acid (HCN) and hydrogen sulfide (H 2 S). Together with Antoine Lavoisier, he developed the modern chemical nomenclature .
In 1789 he discovered the bleaching effect of chlorine . Berthollet, who was the inspector of the dye works and who also published a “Handbook of the Art of Dyeing”, then introduced chlorine or hypochlorite as a bleaching agent into the textile industry. The alkaline solutions, ultimately developed into Eau de Labarraque by Antoine Germain Labarraque , were named " Eau de Javel " ( Javel water ) after the place of production and the Eau de Javel is still today one of the most popular cleaning, bleaching and disinfecting agents sold in France.
His studies on the composition of ammonia (NH 3 ) (1785), hydrocyanic acid (HCN) and hydrogen sulfide (H 2 S) are also important.
Berthollet supported Antoine Lavoisier's refutation of the phlogiston theory - although he was initially a follower of it - which led to the reform of the chemical nomenclature (1787). This was the basis for the development of the chemical terminology still used today.

When there were difficulties with the supply of saltpetre for the manufacture of gunpowder at the beginning of the French Revolution , he became head of a commission that was supposed to ensure the supply of saltpetre to France.
He was also the head of a commission with the aim of improving the methods of iron production , that is, to scientifically define the types of iron alloys (wrought iron) or steels. In this scientific study from the 1780s on pig iron and steel production , the attempt was made to find a systematic explanation based on the degree of reduction , removal of oxygen, of the ores and their subsequent alloying or combination with carbon.
In 1794 he became professor of chemistry at the École polytechnique , see also Chemistry in Modern Times . Four years later, in 1798, as a member of a group of scientists, he accompanied Napoleon Bonaparte (Napoleon I) on a trip to Egypt ( Egyptian expedition ).
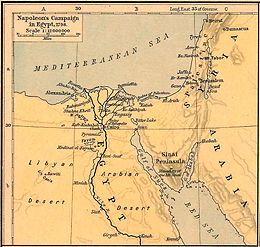
The Egypt expedition led him a. a. to the soda lakes (see bitter lake on the map opposite) where he made some important observations that led him to further considerations. Claude Louis Berthollet recognized that sodium carbonate (Na 2 CO 3 ) deposited as salt crusts on the shores of these lakes, which often dry out in hot periods. He constructed the reaction as follows:
2 NaCl (x) + CaCO 3 (y) → Na 2 CO 3 + CaCl 2
(here in a formulas not available until later generations of chemists, see Jöns Jakob Berzelius (1779–1848))
The decisive question that Berthollets tried to answer was why the reaction proceeded in the direction of the end products, with the known reactants ( i.e. in the direction of Na 2 CO 3 , CaCl 2 ) although, according to the affinity theory, the reaction would only go in the other direction should run. Berthollet pursued this thought further and concluded that the direction of the reaction is influenced by external parameters. He named the mass of the substances involved in the reaction as such a parameter - in modern terms, the concentration or activity . Berthollet published his findings in his books Recherches sur le lois de l'affinité (1801) and Essai de statique chimique (1803).
The chemical processes at Lake Natron would be explained in modern terms as follows: As with all chemical reactions, there is an equilibrium.
Although this is far to the left under standard conditions, if the sodium carbonate (Na 2 CO 3 ) is continuously removed from the equilibrium through crystallization - precisely through the formation of crusts on the bank - the equilibrium shifts to the right, i.e. in the direction of the Products. In 1803 he published the latter, important work "Essai de statique chimique" (German attempt at chemical statics ). This and his other theoretical work on chemical affinity established his importance as a researcher. Berthollet recognized that the proportions for chemical reactions play a decisive role, but he was of the opinion that the composition of a reaction product depends on the conditions. In addition, Berthollet was involved with another French chemist Proust (1754-1826) in a long-term dispute about the validity of the law of constant proportions .
In his work Essai de statique chimique he also included the tables of equivalent measures by Jeremias Benjamin Richter and Ernst Gottfried Fischer and made them known to wider circles.
While this has been refuted by Proust's law of constant proportions (a certain chemical compound has a certain molecular formula and therefore a certain composition), there are in fact some non-molecular compounds that show some variability in composition (e.g. in the iron oxide Fe 1− x O with 0.90 ≤ x ≤ 0.95). Such a non- stoichiometric compound is therefore also called bertholloid . In addition, his view that temperature , pressure and concentration affect the product composition is correct in terms of different product concentrations, such as e.g. B. shows the law of mass action .
Berthollet also played a major role in the development of titration .
Taxonomic honor
The plant genus Bertholletia (the best known representative of the genus is the Brazil nut ) was named in honor of Berthollet.
Works (selection)
- Dissertatio medica (...) Joanne-Baptisa Le Roux des Tillets (ie Joanne-Jacobo Le Roux des Tillets) (...) praeside. De lacte animalium medicamentoso. Quillau, Paris 1779.
- Research on the lois de l'affinité. 1801.
- Essai de statique chimique. 1803.
- Elements de L'Art de La Teinture. Didot, Paris 1804.
- Observations on the natron.
- with Antoine Laurent de Lavoisier and Guyton de Morveau : Système des connaissances chimiques et de leurs applications aux phénomènes de la nature et de l'art. 6 volumes. Paris 1801. (German in excerpt from F. Wals, Königsberg 1801–1803, 4 volumes)
- Méthode De Nomenclature Chimique. Paris 1787. (German: Method of chemical nomenclature for the anti-inflammatory system by Morveau, Lavoisier, Berthollet and de Fourcroy. ) Nachdr. D. Vienna 1793 edition: Olms, Hildesheim 1978, ISBN 3-487-06450-2 ; English translation: Large summary table of the nomenclature. (1788), online access
literature
- Michelle Sadoun-Goupil: Le chimiste Claude-Louis Berthollet, 1748–1822: sa vie, son œuvre . J. Vrin, Paris 1977.
- Michelle Sadoun-Goupil: Science pure et science appliquée dans l'oeuvre de Claude-Louis Berthollet. In: Revue d'histoire des sciences. Volume 27, Numbers 27-2, 1974, pp. 127-145 (online) .
- Michelle Sadoun-Goupil (ed.): Claude-Louis Berthollet, Revue de l'Essai de Statique chimique, édition critique . Ecole Polytechnique, Palaiseau 1980.
- Barbara Whitney Keyser: Between science and craft: The case of berthollet and dyeing. In: Annals of Science. Volume 47, number 3, 1990, pp. 213-260, doi: 10.1080 / 00033799000200211 .
- Patrice Bret: L'État, l'armée, la science. L'invention de la recherche publique en France (1763-1830) . Presses Universitaires de Rennes, Rennes 2002.
Web links
- Literature by and about Claude-Louis Berthollet in the catalog of the German National Library
- Literature by and about Claude-Louis Berthollet in the SUDOC catalog (Association of French University Libraries)
- Entry on Berthollet, Claude Louis (1748–1822), Count in the Archives of the Royal Society , London
Individual evidence
- ↑ P. Lemay, RE Oesper: Claude Louis Berthollet (1748-1822). In: J. Chem. Educ. 23 (4), 1946, p. 158.
- ↑ Genealogy M. Baur
- ^ P. Lemay: Berthollet et la Société d'Arcueil. In: Revue d'histoire de la pharmacie. Volume 21, Numéro 84, 1933, pp. 191–193.
- ^ List of members since 1666: Letter B. Académie des sciences, accessed on September 18, 2019 (French).
- ↑ Holger Krahnke: The members of the Academy of Sciences in Göttingen 1751-2001 (= Treatises of the Academy of Sciences in Göttingen, Philological-Historical Class. Volume 3, Volume 246 = Treatises of the Academy of Sciences in Göttingen, Mathematical-Physical Class. Series 3rd volume 50). Vandenhoeck & Ruprecht, Göttingen 2001, ISBN 3-525-82516-1 , p. 38.
- ↑ Patrimoine des Communes des France ( Memento des Originals from January 18, 2012 in the Internet Archive ) Info: The archive link has been inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ^ Biographical Index: Former RSE Fellows 1783–2002. Royal Society of Edinburgh, accessed October 9, 2019 .
- ^ P. Lemay: Berthollet et la Société d'Arcueil. In: Revue d'histoire de la pharmacie. Volume 21, Numéro 84, 1933, p. 193.
- ↑ Topic Topos. Heritage of France ( Memento des Originals of March 27, 2013 in the Internet Archive ) Info: The archive link has been inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 101st edition. Walter de Gruyter, Berlin 1995, ISBN 3-11-012641-9 .
- ↑ Alphons Oppenheim: History of the chemical theories from Lavoisier up to our time. ( Full text online at archive.org)
| personal data | |
|---|---|
| SURNAME | Berthollet, Claude-Louis |
| ALTERNATIVE NAMES | Berthollet, Claude Louis comte |
| BRIEF DESCRIPTION | French chemist and doctor |
| DATE OF BIRTH | December 9, 1748 |
| PLACE OF BIRTH | Talloires |
| DATE OF DEATH | November 6, 1822 |
| Place of death | Arcueil near Paris |