Linde process

The Linde process is a technical method for gas separation developed by Carl von Linde in 1895 , which enables the liquefaction of gas mixtures such as air and individual atmospheric gases such as oxygen, nitrogen and argon (noble gases) in large quantities and, in this sense, the generation of cold in the temperature range from 77 to 100 Kelvin (K).
history
Although initially only used for academic purposes, it was first used in industry as early as 1902 as an important part of the air separation plant (technical abbreviation: LZA) also developed by Carl von Linde. Even today, air separation plants are used on a large scale to extract gaseous and liquid oxygen (GOX and LOX (from liquid oxygen) - as technology codes), nitrogen (GAN and LIN ) and noble gases . In contrast, the Linde process in its original structure is no longer used to generate cold, since more efficient technical implementations (reciprocating piston expanders or expansion turbines ) have been developed. Their cold generation is no longer based on the pure Joule-Thomson effect of the original Linde process, but on achieving as adiabatic cooling as possible while obtaining useful mechanical energy from the expanding gas. In the original Linde process, on the other hand, or with the pure throttle-expansion-isenthalpic Joule-Thomson effect, this energy is not only not used, it even remains in the process as throttle frictional heat that is harmful to the process. This z. B. a helium liquefaction without pre-cooling with the original pure throttle-expansion-isenthalpic Linde process is impossible because of the low inversion temperature of helium, but with the improved process, because the removal of useful mechanical energy during the expansion of a gas under adiabatic conditions regardless of Gas type and gas temperature always lead to a cooling. The use of the Joule-Thomson effect is therefore still in use.
principle
The isenthalpic expansion of a real gas in a throttle is accompanied by a change in its temperature ( Joule-Thomson effect ). The abstract model of the ideal gas does not show this effect. Whether the temperature change occurs in the form of cooling or heating depends on whether the temperature has fallen below the inversion temperature (i.e. the temperature at which the Joule-Thomson coefficient of the gas undergoes a change in sign). If the system is above the inversion temperature, the gas heats up during expansion (more precisely: isenthalpic expansion, the enthalpy does not change due to the change in volume), lower temperatures result in cooling; this effect is used in the Linde process. (Note: an isenthalpic expansion occurs according to the 1st law of thermodynamics in a throttle if the change in the potential and kinetic energy of the flowing fluid from throttle inlet to throttle outlet can be neglected and it can be assumed that the throttle is adiabatically isolated .)
In order to achieve the boiling temperature, which is low for many gases (for oxygen −183 ° C, for nitrogen −196 ° C), the expanded gas is used in the counterflow principle to precool the compressed gas.
application
The Linde process was previously used to cool atmospheric gases oxygen , nitrogen , argon and other noble gases until they liquefy.
Air liquefaction
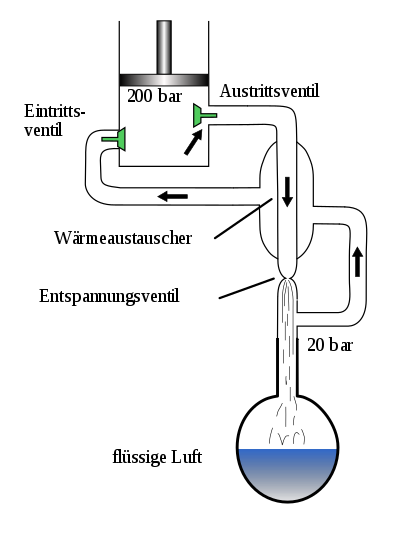
A compressor compresses the air to a pressure of approx. 200 bar . Their temperature increases by approx. 45 Kelvin, for example from +20 ° C to approx. +65 ° C. The compressed, heated air is then pre-cooled in a first heat exchanger and the temperature is returned to the range of ambient temperature. In the process, heat is released from the air liquefaction system into the environment. The air is first washed and freed from water vapor, dust, hydrocarbons, nitrous oxide and carbon dioxide using a molecular sieve . Hydrocarbons and nitrous oxide can cause deflagration or even an explosion in the rectification column . The air is then expanded using a turbine, whereby the temperature of the air drops to just before the liquefaction point. The air is then passed through a throttle valve, where the air then reaches the condensation point (approx. −170 ° C).
The engineer Fränkl succeeded in replacing the countercurrent recuperators with regenerators. These can be built much smaller, cheaper and more efficient than counterflow tube bundle heat exchangers . This invention was taken over by Linde AG and marketed under the name Linde-Fränkl-Verfahren. The process with regenerators was used successfully until around 1990, when a newer technology emerged which again included recuperative countercurrent plate heat exchangers with upstream adsorptive drying and cleaning.
Liquid air has a density of about 875 kg / m³ (0.875 g / cm³). In an open vessel under atmospheric pressure, it assumes a temperature of −194.25 ° C = 78.9 K. It boils in the process, so that its low temperature is maintained, as this removes heat of vaporization from the liquid air . Since oxygen and nitrogen have different boiling temperatures, the nitrogen evaporates more quickly and the boiling temperature of the remaining liquid rises slightly, up to 81.7 K. The amount of boiling air is regulated in such a way that the heat supplied by conduction or radiation is equal to the evaporation heat used is. Depending on the size and insulation of the container, the liquid air can be retained for a few hours to many days. However, liquid air must never be stored in closed containers without safety devices and appropriate design, as the internal pressure, which increases gradually due to the heating, will cause it to burst.
Fractionating the liquefied air
Liquid air can be broken down into its constituent parts by means of fractionation, in which the different boiling points of the individual air constituents are used. However, the boiling points of oxygen and nitrogen are very close together. A rectification column is therefore used : the liquid air runs downwards over several rectification trays in countercurrent to the rising gas. It absorbs oxygen from the gas and releases nitrogen. The rectification is carried out at a pressure of approx. 5-6 bar. This makes the liquid more oxygenated and the gas more nitrogenous.
Liquefaction of hydrogen and helium
In order to be able to use the Linde process for hydrogen and helium liquefaction, these gases must first be pre-cooled below the inversion temperature. This is usually done with liquid air. The liquid helium finally obtained boils under atmospheric pressure at 4.2 K. This is the lowest boiling point of all elements. By pumping out the helium gas above the boiling helium, heat of evaporation is extracted from the latter, so that its temperature can be further reduced. However, since the vapor pressure drops very sharply with the temperature, no lower temperature than 0.84 K is reached with this process; it includes the vapor pressure 0.033 mbar.
Physical basics
The Linde method is based on the Joule-Thomson effect: in the ideal gas, the particles do not interact with one another, which is why the temperature of the ideal gas does not depend on the volume. In the case of real gases, on the other hand, there are interactions that are described with the help of the Van der Waals equation . The energy content of the real gas also changes with adiabatic (without heat exchange) expansion without any external work being done. This can be proven by the change in temperature.
If you connect two gas containers with a porous wall and use a piston to slowly push the gas under pressure in room 1 through this membrane, which serves to prevent eddies and jet formation, in room 2, which is under a constant but lower pressure than room 1 then there is a small temperature difference between the two rooms. With carbon dioxide it is about 0.75 K per bar of pressure difference, with air about 0.25 K.
This can be explained if you consider that the volume in room 1 has been removed. The piston has done the work for the gas . The amount of gas appears in space 2 and has to do the work against the piston. The difference in work has benefited the gas as internal energy.
- or.
The enthalpy remains constant. In Van der Waals gas is the internal energy , where is the number of degrees of freedom of a particle.
Taking the Van der Waals equation into account, this results in :
Because the enthalpy is retained, the following applies to the total differential :
Reshaped after the change in temperature results:
The counter is positive at high temperature. It changes its sign at the inversion temperature .
So the critical temperature for a van der Waals gas is .
Above , a gas heats up when it is released, below it cools down. For carbon dioxide and air is well above room temperature, for hydrogen, however, at −80 ° C.
A high value of the Van der Waals constant therefore has the effect that the temperature drops sharply when the real gas is expanded. This is logical, because when the volume increases, the molecules move away from each other and have to work against the forces of attraction characterized by . This work reduces the kinetic energy of the molecules and thus the temperature of the gas.
Alternative procedures
Two newer processes are used for the more cost-effective production of nitrogen and oxygen in a purity adapted to requirements:
- Membrane gas separation ( English membrane gas separation (MGS) ): diffusion through the hollow fiber membrane may be high purity nitrogen and oxygen of up to 40% enrichment degree deliver compressed air.
- By means of pressure swing adsorption ( English pressure swing adsorption (PSA) ) on molecular sieves , CMS - carbon molecular sieve nitrogen or zeolites - "Zeo molecular sieve" is oxygen can be decomposed air via pressure swing in two pressure vessels.
literature
- Christian Gerthsen , Hans Otto Kneser , Helmut Vogel : Physics: a textbook for use in addition to lectures . 14th edition. Springer, Berlin / Heidelberg 1982, ISBN 3-540-11369-X , chapters 5.6.6 and 5.6.7.
- Georg Veranneman: Technical gases. Production, distribution, application (= Library of Technology , Volume 10), 4th edition, Verlag Moderne Industrie, Landsberg am Lech 2000, ISBN 3-478-93229-7 .
Web links
- Linde videos that visually show the air separation process:
- from the plant perspective: https://www.youtube.com/watch?v=-mUKww4iPgw
- from the gas perspective: https://www.youtube.com/watch?v=flvW0vTQ1NM
- Information on helium liquefiers from Linde
- SVGs: Separation column , plant using the low-pressure process
- Video: JOULE-THOMSON effect and LINDE method - How do you create liquid air? . Jakob Günter Lauth (SciFox) 2013, made available by the Technical Information Library (TIB), doi : 10.5446 / 15652 .