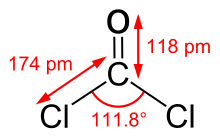
Phosgene
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Phosgene | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | COCl 2 | ||||||||||||||||||
| Brief description |
|
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 98.92 g mol −1 | ||||||||||||||||||
| Physical state |
gaseous |
||||||||||||||||||
| density |
|
||||||||||||||||||
| Melting point |
−127.76 ° C |
||||||||||||||||||
| boiling point |
7.6 ° C |
||||||||||||||||||
| Vapor pressure |
|
||||||||||||||||||
| solubility |
decomposes in water |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| MAK |
DFG / Switzerland: 0.1 ml m −3 or 0.41 mg m −3 |
||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| Thermodynamic properties | |||||||||||||||||||
| ΔH f 0 |
−219.1 kJ / mol |
||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Phosgene is the common name for carbon dioxide dichloride or carbonyl chloride, COCl 2 , the dichloride of carbonic acid . As diphosgene or triphosgene compounds of the composition are C 2 O 2 Cl 4 and C 3 O 3 Cl 6 designates which, although used as synthetic equivalents of phosgene, however, not in a narrow sense oligomers are the same.
The substance, which is extremely toxic as a gas, is only used in industry in hermetically sealed circuits . In some wars it was used as a deadly chemical warfare agent. For a long time, however, it has been outlawed by the international chemical weapons convention.
history
Phosgene was discovered in 1812 by John Davy , the younger brother of Sir Humphry Davy . The name phosgene (Greek: generated by light; cf. biogenic , anthropogenic ) comes from the photo-induced addition of chlorine gas to carbon monoxide that he carried out .
The use of phosgene as a chemical gas warfare agent ( Grünkreuz ) was responsible for the majority of the approximately 90,000 gas deaths in the First World War .
properties
Phosgene is a very toxic gas, the smell of which can be described as sweetish rotten (rotting banana peel or damp hay ). This is very characteristic and easy to identify.
Phosgene is readily soluble in organic solvents (e.g. benzene , toluene , chlorobenzenes and others); it dissolves in water with gradual decomposition to carbon dioxide and hydrochloric acid , which is why anhydrous organic solvents are usually used for the synthesis or conversion of phosgene.
- Hydrolysis of phosgene to carbon dioxide and hydrogen chloride
Phosgene has a heat of vaporization of 24.38 kJ mol −1 .
Toxicity
Since phosgene is poorly soluble in water, it passes by inhalation to the blood-air barrier in the alveoli (alveoli). Due to the moisture present there, it gradually decomposes in the alveoli into carbon dioxide and hydrochloric acid . The hydrochloric acid corrodes the lung tissue and the alveoli. After two to three hours, this leads to an excruciating cough , cyanosis and pulmonary edema and is usually fatal. Death usually occurs by suffocating while fully conscious. High doses can also lead to death within seconds or minutes because the phosgene molecules react in large numbers with the amino acids in the alveolar walls and prevent the exchange of oxygen. Unlike mustard gas , phosgene is only absorbed through the lungs, not through the skin.
Accidents
- On May 20, 1928, phosgene leaked from a leaky tank car on the premises of the Stoltzenberg chemical factory ( Hamburg ). 10 people died and about 150 (according to other sources about 300) were injured.
- On March 14, 2008, in a laboratory at the Technical University of Munich in Garching, a hose from a test facility came loose due to a defect and phosgene escaped. Two people were brought to the intensive care unit because pulmonary edema had formed, and 38 other people were examined in the hospital as a precaution.
- On January 23, 2010 at the DuPont Chemical Facility in Belle, West Virginia, the sudden rupture of a braided steel hose attached to a phosgene tank resulted in the release of phosgene. An employee was exposed to the toxic gas-air mixture and succumbed to the effects of the exposure one day later.
Manufacturing
Phosgene is produced from carbon monoxide and chlorine under the catalytic influence of activated carbon :
The strongly exothermic reaction has to be cooled with great effort, since temperatures in the "hotspot" of up to 600 ° C arise. Carbon monoxide is used in excess to guarantee the complete conversion of the chlorine and thus to prevent a chlorine iron fire (exothermic reaction of chlorine and iron at temperatures above 170 ° C). Residual amounts of methane from carbon monoxide production react to form carbon tetrachloride . The carbon monoxide must absolutely be free of hydrogen so that no chlorine gas is generated.
Phosgene can certainly also during the combustion of chlorine-containing plastics (eg PVC ) in the presence of metal and coal (z. B. solder old copper lines of refrigeration systems) and in combustion of 1 January 2010, banned refrigerant R22 arise. On a laboratory scale, it can also be made from carbon tetrachloride and fuming sulfuric acid ("oleum"):
use
Phosgene was used in the military as a chemical gas warfare agent:
Germany was the first nation to use it as a 5% admixture to chlorine gas on May 31, 1915 (near Ypres in the Second Battle of Flanders ). On February 22, 1916, France used phosgene in its pure form. Phosgene is ascribed the largest proportion of all gas injuries. Later, the warfare agents were poison gas grenades fired, mostly alternating with poison gas grenades with other toxins ( " Colorful shooting ").
Phosgene is an important synthetic building block, for example for the production of carboxylic acid chlorides (whereby the less problematic thionyl chloride is usually used here) and polyurethanes (via isocyanates ) as well as polycarbonate plastics (abbreviation: PC, for example for the production of CDs) and other intermediate products, which are then used to produce Medicines , dyes and insecticides are used.
Because phosgene is so dangerous, in the chemical industry today it is predominantly produced in the same plant in which it is consumed, so that no transport has to take place and the resulting toxic phosgene is converted into harmless secondary products as quickly and completely as possible. As alternatives, liquid diphosgene (trichloromethyl chloroformate) and solid triphosgene (bis- trichloromethyl carbonate) are available, which show a similar reactivity, but are safer to use and easier to store.
See also
- Gas war during the First World War
- List of chemical warfare agents
- Lung warfare agent
- Mask breaker
- Settle fabric ("red cross"), yellow cross , mustache , white cross
- Thiophosgene
literature
- Beyer Hans, Walter Wolfgang: Textbook of organic chemistry . 23. revised and updated edition. S. Hirzel Verlag, Stuttgart / Leipzig 1998, ISBN 3-7776-0808-4 .
- Dominique Lapierre and Javier Moro : Five past twelve in Bhopal. The incredible story of the greatest toxic gas disaster of our time. Europa Verlag, Vienna 2004 (French original edition 2001), ISBN 3-203-79508-6 .
Web links
Individual evidence
- ↑ a b Entry on phosgene. In: Römpp Online . Georg Thieme Verlag, accessed on June 14, 2014.
- ↑ a b c d e f g h i j k l Entry on phosgene in the GESTIS substance database of the IFA , accessed on November 13, 2017(JavaScript required) .
- ↑ Entry on phosgenes in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 75-44-5 or phosgene ), accessed on November 2, 2015.
- ↑ a b Entry on phosgenes in the Hazardous Substances Data Bank , accessed July 27, 2012.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Standard Thermodynamic Properties of Chemical Substances, pp. 5-19.
- ↑ The Little Man's Nuclear Weapon . In: Der Spiegel . No. 39 , 1998 ( online - 26 September 1998 ).
- ^ University of Munich: Police investigate after poison gas accident , Spiegel Online, report from March 16, 2008.
- ^ DuPont Corporation Toxic Chemical Releases | COD. Retrieved August 30, 2018 .
- ↑ Entry on chlorine in the GESTIS substance database of the IFA , accessed on November 16, 2017(JavaScript required) .
- ↑ First World War: "Yellow Cross" should be the ultimate weapon. In: Welt Online . Retrieved July 14, 2017 .