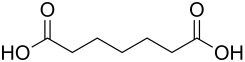
Pimelic acid
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Pimelic acid | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 7 H 12 O 4 | |||||||||||||||||||||
| Brief description |
white crystals |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 160.17 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| density |
1.33 g cm −3 |
|||||||||||||||||||||
| Melting point |
102-105 ° C |
|||||||||||||||||||||
| boiling point |
223 ° C |
|||||||||||||||||||||
| pK s value |
|
|||||||||||||||||||||
| solubility |
poor in water (50 g l −1 at 20 ° C) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Pimelic acid (also heptanedioic acid or 1,5-pentanedicarboxylic acid ) belongs to the homologous series of dicarboxylic acids . The name is also derived from the Greek pimele (= fat). It lies between adipic acid and suberic acid ( suberic acid ). Their salts are called pimelates.
In contrast to adipic acid, pimelic acid has only a very limited area of application in the chemical industry.
properties
As a dibasic acid, pimelic acid dissociates in water in two stages of protolysis . Their octanol-water partition coefficient is log (K OW ) = 0.61.
Biological importance
Pimeloyl-CoA is a component in the biosynthesis of biotin .
Individual evidence
- ↑ a b c d e f g Data sheet pimelic acid (PDF) from Merck , accessed on February 13, 2010.
- ^ A b Fieser and Fieser, Organic Chemistry, 2nd Edition, Verlag Chemie 1982 ISBN 3-527-25075-1 .
- ↑ a b Data sheet Pimelic acid from Sigma-Aldrich , accessed on April 20, 2011 ( PDF ).
- ↑ Roempp Chemie Lexikon 9th Edition 1991 Keywords pimelic
- ↑ Paola Estrada, Miglena Manandhar, Shi-Hui Dong, Jaigeeth Deveryshetty, Vinayak Agarwal, John E. Cronan, Satish K. Nair: The pimeloyl-CoA synthetase BioW defines a new fold for adenylate-forming enzymes. In: Nature Chemical Biology. 13, 2017, pp. 668-674, doi : 10.1038 / nchembio.2359 .
