List of acids
The following list shows a selection of acids . Are listed
- the common common name
- the IUPAC name (the official name)
- the molecular formula or a simplified structural formula
The list does not claim to be complete.
Inorganic acids (selection)
Acids of the noble gases
| element | Oxidation state of the central atom | Common name | formula | Salts |
|---|---|---|---|---|
| xenon | +6 | Xenonic acid | Xenate | |
| +8 | Perxenonic acid | Perxenate |
Acids of halogens
| element | Oxidation state of the halogen atom | Common name | formula | Salts | Remarks |
|---|---|---|---|---|---|
| fluorine | −1 | Hydrofluoric acid | Fluoride | Aqueous solution of hydrogen fluoride | |
| −1 | Hypofluoric acid | Hypofluorites | |||
| chlorine | −1 | hydrochloric acid | Chlorides | Aqueous solution of hydrogen chloride | |
| +1 | Hypochlorous acid | Hypochlorites | |||
| +3 | Chlorous acid | Chlorites | |||
| +5 | Chloric acid | Chlorates | |||
| +7 | Perchloric acid | Perchlorates | |||
| bromine | −1 | Hydrobromic acid | Bromides | ||
| +1 | Hypobromous acid | Hypobromites | |||
| +3 | Brominous acid | Bromites | |||
| +5 | Bromic acid | Bromates | |||
| +7 | Perbromic acid | Perbromate | |||
| Iodine | −1 | Hydriodic acid | Iodides | Aqueous solution of hydrogen iodide | |
| +1 | Hypoiodous acid | Hypoiodite | |||
| +3 | Iodic acid | Iodites | |||
| +5 | Iodic acid | Iodates | |||
| +7 | Periodic acid |
(Metaperiodic acid), (orthoperiodic acid), (triperiodic acid)
|
Periodates | There are different types of periodates because there are several periodic acids . |
Acids of the chalcogens
| element | Oxidation state | Common name | formula | Salts | Remarks |
|---|---|---|---|---|---|
| sulfur | −2 | Hydrogen sulfide | Sulphides | ||
| +2 | Sulfoxylic acid | Sulfoxylates | |||
| −1 / +5 | Thiosulfuric acid | Thiosulfates | |||
| +3 | Dithionic acid | ||||
| +3 / +5 | Disulphurous acid | Disulfites | |||
| +4 | sulphurous acid | Sulfites | Formed by the reaction of sulfur dioxide with water | ||
| +5 | Dithionic acid | Dithionates | |||
| +6 | sulfuric acid | Sulfates | When using sulfur trioxide produced | ||
| +6 | Disulfuric acid | Disulfates | |||
| +6 | Peroxomonosulfuric acid | Peroxomonosulfates | |||
| +6 | Peroxodisulfuric acid | Peroxodisulfates | |||
| selenium | −2 | Hydrogen selenide | Selenides | ||
| +4 | Selenous acid | Selenite | |||
| +6 | Selenic acid | Selenate | |||
| Tellurium | -2 | Tellurium hydrogen | Telluride | ||
| +4 | Telluric acid | Tellurite | |||
| +6 | Telluric acid | Tellurates |
Acids of the nitrogen group
| element | Oxidation state | Common name | formula | Salts | Remarks |
|---|---|---|---|---|---|
| nitrogen | −2 / −3 / −4 | Hydrazoic acid | Azides | If using hydrazine produced | |
| +1 | Hypo-nitrous acid | Hyponitrites | |||
| +3 | Nitrous acid | Nitrites | When using nitrogen dioxide produced | ||
| +5 | nitric acid | Nitrates | |||
| +5 | Peroxo nitric acid | Peroxonitrates | |||
| phosphorus | +1 | Phosphinic acid | Phosphinates | ||
| +3 | Phosphonic acid | Phosphonates | |||
| +5 | Hypodiphosphonic acid | Hypodiphosphonates | |||
| +5 | Diphosphonic acid | Diphosphonates | |||
| +5 | phosphoric acid | Phosphates | Is using phosphorus pentoxide produced | ||
| +4 | Hypodiphosphoric acid | Hypodiphosphates | |||
| +5 | Diphosphoric acid | Diphosphates | |||
| +5 | Peroxophosphoric acid | Peroxophosphates | |||
| +5 | Peroxodiphosphoric acid | Peroxodiphosphates | |||
| arsenic | +3 | Arsenic acid | Arsenites | ||
| +5 | Arsenic acid | Arsenates | |||
| antimony | +3 | Antimony acid | Antimonites | ||
| +5 | Antimonic acid | Antimonates |
Acids of carbon and boron group
| element | Oxidation state | Common name | formula | Salts | Remarks |
|---|---|---|---|---|---|
| carbon | +4 | carbonic acid | Carbonates | Formed by the reaction of carbon dioxide with water | |
| Silicon | +4 | Metasilicic acid | Metasilicates | ||
| +4 | Orthosilicic acid | Orthosilicates | |||
| +4 | Orthodilicic acid | Orthodisilicates | |||
| boron | +3 | Boric acid | Borates |
Acids of the transition metals
Other acids
| Common name | formula | Salts |
|---|---|---|
| Amidosulfonic acid | H 2 N-SO 2 -OH | Amidosulfonates |
| Hydrogen cyanide | HCN | Cyanide |
| Cyanic acid | HOCN | Cyanates |
| Fulmic acid / pop acid | HCNO | Fulminates |
| Isocyanic acid | HNCO | Cyanates |
| Isofulmic acid / isopallic acid | HONC | Fulminates |
| Aqua regia | Mixture of 3 parts hydrochloric acid and 1 part nitric acid |
Organic acids (selection)
Short-chain aliphatic carboxylic acids and derivatives
| Initial alkane | saturated carboxylic acid | unsaturated carboxylic acids | saturated dicarboxylic acids | unsaturated dicarboxylic acids | Oxygenated derivatives |
|---|---|---|---|---|---|
| Methane (CH 4 ) |
Formic acid HCOOH |
- | - | - | - |
| Ethane (C 2 H 6 ) |
Acetic acid H 3 C-COOH |
- |
Oxalic acid HOOC-COOH |
- |
Glycolic acid HOCH 2 -COOH glyoxalic acid O = CH-COOH |
| Propane (C 3 H 8 ) |
Propionic acid H 3 C-CH 2 -COOH |
Acrylic acid H 2 C = CH-COOH |
Malonic acid HOOC-CH 2 -COOH |
- |
Lactic acid H 3 C-CH (OH) -COOH tartronic acid HOOC-CH (OH) -COOH |
| n -butane (C 4 H 10 ) |
Butyric acid H 3 C- (CH 2 ) 2 -COOH |
Crotonic acid H 3 C-CH = CH-COOH (cis position) Isocrotonic acid H 3 C-CH = CH-COOH (trans position) vinylacetic acid H 2 C = CH-CH 2 -COOH |
Succinic acid HOOC- (CH 2 ) 2 -COOH |
Maleic acid HOOC-CH = CH-COOH (cis position) Fumaric acid HOOC-CH = CH-COOH (trans position) |
Gamma-hydroxybutyric acid HO- (CH 2 ) 3 -COOH Malic acid HOOC-CH (OH) -CH 2 -COOH Tartaric acid HOOC-CH (OH) -CH (OH) -COOH Oxaloacetic acid HOOC-CH 2 -CO-COOH Squaric acid C 4 H 2 O 4 |
| n -pentane (C 5 H 12 ) |
Valeric acid H 3 C- (CH 2 ) 3 -COOH |
Allylacetic acid H 2 C = CH- (CH 2 ) 2 -COOH |
Glutaric acid HOOC- (CH 2 ) 3 -COOH |
- |
α-ketoglutaric acid HOOC-CH 2 -CH 2 -CO-COOH citric acid HOOC-CH 2 -C (OH) (COOH) -CH 2 -COOH isocitric acid HOOC-CH (OH) -CH (COOH) -CH 2 -COOH aconitic acid HOOC-CH = CH (COOH) -CH 2 -COOH |
| n -hexane (C 6 H 14 ) |
Caproic acid H 3 C- (CH 2 ) 4 -COOH |
Sorbic acid H 3 C-CH = CH-CH = CH-COOH (trans position) |
Adipic acid HOOC- (CH 2 ) 4 -COOH |
- |
Gluconic acid HO-CH 2 - (CH (OH)) 4 -COOH |
| n -Heptane (C 7 H 16 ) |
Enanthic acid H 3 C- (CH 2 ) 5 -COOH |
- |
Pimelic acid HOOC- (CH 2 ) 5 -COOH |
- | - |
| n- octane (C 8 H 18 ) |
Caprylic acid H 3 C- (CH 2 ) 6 -COOH |
- |
Suberic acid HOOC- (CH 2 ) 6 -COOH |
- | - |
| n -Nonane (C 9 H 20 ) |
Pelargonic acid H 3 C- (CH 2 ) 7 -COOH |
- |
Azelaic acid HOOC- (CH 2 ) 7 -COOH |
- | - |
| n -decane (C 10 H 22 ) |
Capric acid H 3 C- (CH 2 ) 8 -COOH |
- |
Sebacic acid HOOC- (CH 2 ) 8 -COOH |
- | - |
Long chain aliphatic carboxylic acids and derivatives
Other carboxylic acids and derivatives
amino acids
- Only amino acids with an acidic character are given here.
| Common name (IUPAC name) | formula | Salts |
|---|---|---|
| Aspartic acid | HOOC-CH 2 -CH (NH 2 ) -COOH | Aspartates |
| Carbamic acid | H 2 N-COOH | Carbamates |
| Glutamic acid | HOOC-CH 2 -CH 2 -CH (NH 2 ) -COOH | Glutamates |
Further carboxylic acid derivatives
- Further derivatives of carboxylic acids which contain foreign atoms such as halogens, sulfur or phosphorus are specified here.
| Common name (IUPAC name) | formula | Salts |
|---|---|---|
| Chloroacetic acid | CH 2 Cl-COOH | Monochloroacetate |
| Fluoroacetic acid | CH 2 F-COOH | Monofluoroacetate |
| Trichloroacetic acid | Cl 3 C-COOH | Trichloroacetate |
| Trifluoroacetic acid | F 3 C-COOH | Trifluoroacetate |
Alphabetical list of common names (selection)
- Note: Very long IUPAC names have been partially omitted here. They can be found in the article on the respective acid.
| Common name | IUPAC name | Chopstick model | Structural formula | formula | Characteristic elements | Acid constants (pK s ) | Salts | Remarks |
|---|---|---|---|---|---|---|---|---|
| Abietic acid | Abieta-7,14-diene-19-carboxylic acid |
|

|
C 20 H 30 O 2 | carbon | Abietates | Part of tree sap | |
| Acetylsalicylic acid | 2- (acetyloxy) benzoic acid |
|
|
HOOC-C 6 H 4 -COOCH 3 | carbon | 3.49 | Acetyl salicylates | The medicinal ingredient aspirin. A derivative of salicylic acid and benzoic acid . |
| Acrylic acid | Propenoic acid |

|

|
H 2 C = CH-COOH | carbon | 4.26 | Acrylates | A monounsaturated carboxylic acid |
| Adipic acid | Hexanedioic acid |
|

|
HOOC- (CH 2 ) 4 -COOH | carbon | 4.43; 5.42 | Adipates | A dicarboxylic acid |
| Malic acid | 2-hydroxybutanedioic acid |

|
|
HOOC-CH 2 -CH (OH) -COOH | carbon | 3.46; 5.10 | Malates | A dicarboxylic acid |
| Alendronic acid | 4-amino-1-hydroxybutylidene diphosphonic acid |

|

|
C 4 H 13 NO 7 P 2 | Carbon , nitrogen , phosphorus | 2.72 | Alendronate | |
| Formic acid | Methanoic acid |
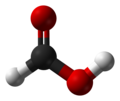
|
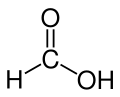
|
HCOOH | carbon | 3.77 | Formates | The simplest carboxylic acid and alkanoic acid |
| Amidosulfonic acid | Amidosulfuric acid |
 
|
|
H 2 N-SO 2 -OH | Nitrogen , sulfur | 1.0 | Amidosulfonates | Comes at standard conditions only as zwitterion + H 3 N-SO 3 - before |
| Antimony acid | H 3 SbO 3 | antimony | Antimonites | |||||
| Antimonic acid | Hexahydroxoantimony (V) acid |
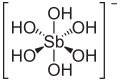
|
H [Sb (OH) 6 ] | antimony | 2.55 | Antimonates | ||
| Arachidonic acid | 5,8,11,14-eicosatetraenoic acid |

|
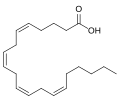
|
C 20 H 32 O 2 | carbon | 4,752 | ||
| Arachidic acid | Eicosanoic acid |
|
H 3 C- (CH 2 ) 18 -COOH | carbon | Arachinoates | A saturated fatty acid and alkanoic acid | ||
| Arsenic acid | Trihydrogen arsenite |

|

|
H 3 AsO 3 | arsenic | Arsenites | ||
| Arsenic acid | Trihydrogen arsenate |

|

|
H 3 AsO 4 | arsenic | 2.26; 6.76; 11.29 | Arsenates | |
| Ascorbic acid | (5R) -5 - [(1S) -1,2-dihydroxyethyl] -
3,4-dihydroxy-5-hydrofuran-2-one |

|

|
C 6 H 8 O 6 | carbon | 4.25 | Ascorbates | Also called vitamin C. |
| Barbituric acid | 2,4,6-trihydroxypyrimidine |

|

|
C 4 H 4 N 2 O 3 | Carbon , nitrogen | 4.01 | Barbiturates | A derivative of urea |
| Behenic acid | Docosanoic acid |
|
H 3 C- (CH 2 ) 20 -COOH | carbon | Behenate | A saturated fatty acid and alkanoic acid | ||
| Benzoic acid | Benzenecarboxylic acid |

|

|
C 6 H 5 COOH | carbon | 4.2 | Benzoates | A derivative of benzene |
| Succinic acid | Butanedioic acid |

|

|
HOOC- (CH 2 ) 2 -COOH | carbon | 4.16; 5.61 | Succinates | A dicarboxylic acid |
| Prussic acid | Hydrogen cyanide |

|
|
HCN | Carbon , nitrogen | 9.40 | Cyanide | |
| Bicinchoninic acid | 2,2'-biquinoline-4,4'-dicarboxylic acid |

|
C 20 H 12 N 2 O 4 | Carbon , nitrogen | A dicarboxylic acid | |||
| Boric acid | Trihydrogen borate |
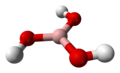
|
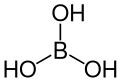
|
H 3 BO 3 | boron | 9.24; 12.4; 13.3 | Borates | |
| Pyruvic acid | 2-oxopropanoic acid |
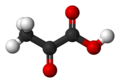
|

|
CH 3 -CO-COOH | carbon | 2.49 | Pyruvates | The simplest keto acid |
| Brominous acid | Hydrogen bromite |

|

|
HBrO 2 | bromine | Bromites | ||
| Bromic acid | Hydrogen bromate |

|

|
HBrO 3 | bromine | −2.0 | Bromates | |
| Hydrobromic acid | Hydrogen bromide |

|
HBr (aq) | bromine | −9.0 | Bromides | Aqueous solution of hydrogen bromide . A super acid . | |
| Butyric acid | Butanoic acid |

|

|
H 3 C- (CH 2 ) 2 -COOH | carbon | 4.82 | Butyrate | A saturated fatty acid and alkanoic acid |
| Capric acid | Decanoic acid |
|
|
H 3 C- (CH 2 ) 8 -COOH | carbon | 4.9 | Decanoate | A saturated fatty acid and alkanoic acid |
| Caproic acid | Hexanoic acid |
|
|
H 3 C- (CH 2 ) 4 -COOH | carbon | 4.85 | Hexanoates | A saturated fatty acid and alkanoic acid |
| Caprylic acid | Octanoic acid |
|
|
H 3 C- (CH 2 ) 6 -COOH | carbon | 4.89 | Caprate | A saturated fatty acid and alkanoic acid |
| Carbamic acid | Aminomethanoic acid |

|
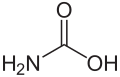
|
H 2 N-COOH | Carbon , nitrogen | Carbamates | An amino acid | |
| Cerotic acid | Hexacosanoic acid |

|
|
C 25 H 51 COOH | carbon | Hexacosanoates | A saturated fatty acid and alkanoic acid | |
| Quinic acid | 1,3,4,5-tetrahydroxy-cyclohexane-1-carboxylic acid |
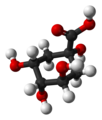
|
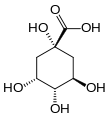
|
C 6 H 7 (OH) 4 COOH | carbon | |||
| Chloroacetic acid | Monochloroethanoic acid |

|

|
H 2 ClC-COOH | Carbon , chlorine | 2.87 | Monochloroacetate | |
| Chlorous acid | Hydrogen chlorite |

|

|
HClO 2 | chlorine | 1.97 | Chlorites | |
| Chloric acid | Hydrogen chlorate |

|

|
HClO 3 | chlorine | −2.7 | Chlorates | |
| Chorismic acid | (3 R ) - trans - (1-carboxyvinyloxy) -4-hydroxy-1,5-cyclohexadiene-1-carboxylic acid |

|
C 10 H 10 O 6 | carbon | Chorismates | |||
| Chromic acid | Dihydrogen chromate |

|

|
H 2 CrO 4 | chrome | −0.8; 1.6 | Chromates | |
| Citric acid | 3-carboxy-3-hydroxypentane-1,5-diacid |

|
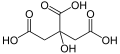
|
HOOC-CH 2 -C (OH) (COOH) -CH 2 -COOH | carbon | 3.13; 4.76; 6.4 | Citrates | A tricarboxylic acid |
| Clavulanic acid | - |
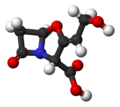
|

|
C 8 H 9 NO 5 | Carbon , nitrogen | Clavulanates | ||
| Cyanic acid | Hydrogen cyanate |

|

|
HOCN | Carbon , nitrogen | Cyanates | ||
| Dichromic acid | Dihydrogen dichromate |

|
H 2 Cr 2 O 7 | chrome | Dichromates | |||
| Diphosphonic acid | Hydrogen diphosphonate | H 4 P 2 O 5 | phosphorus | Diphosphonates | ||||
| Diphosphoric acid | Hydrogen diphosphates |

|

|
H 4 P 2 O 7 | phosphorus | 1.52; 2.36; 6.60; 9.25 | Diphosphates | |
| Disulfuric acid | Dihydrogen disulfate |

|

|
H 2 S 2 O 7 | sulfur | Disulfates | ||
| Ellagic acid | - |

|

|
C 14 H 6 O 8 | carbon | |||
| Erucic acid | cis -13-docosenoic acid |
|
H 3 C- (CH 2 ) 7 -CH = CH- (CH 2 ) 11 -COOH | carbon | A monounsaturated fatty acid | |||
| acetic acid | Ethanoic acid |

|

|
CH 3 COOH | carbon | 4.76 | Acetates | An alkanoic acid |
| Fluoroacetic acid | Monofluoroethanoic acid |
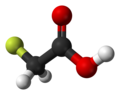
|
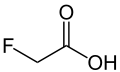
|
CH 2 F-COOH | Carbon , fluorine | 2.59 | Monofluoroacetate | |
| Fluorosulfonic acid |

|

|
HSO 3 F | Sulfur , fluorine | −10.0 | A super acid | ||
| Hydrofluoric acid | Hydrofluoric acid / hydrogen fluoride |

|
HF (aq) | fluorine | 3.17 | Fluoride | Aqueous solution of hydrogen fluoride | |
| Fumaric acid | (2 E ) -But-2-enedioic acid |

|

|
HOOC-CH = CH-COOH | carbon | 3.02; 4.38 | Fumarates | A monounsaturated dicarboxylic acid |
| Fusaric acid | 5-butyl-pyridine-2-carboxylic acid |
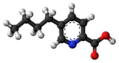
|

|
C 10 H 13 NO 2 | Carbon , nitrogen | |||
| Fusidic acid | - |

|
C 31 H 48 O 6 | carbon | Fusidate | |||
| Gallic acid | 3,4,5-trihydroxybenzoic acid |

|

|
C 7 H 6 O 5 | carbon | Gallate | ||
| Gamma aminobutyric acid | 4-aminobutanoic acid |

|
|
H 2 N- (CH 2 ) 3 -COOH | Carbon , nitrogen | 4.05 | An amino acid | |
| Gamma-hydroxybutyric acid | 4-hydroxybutanoic acid |

|
|
HO- (CH 2 ) 3 -COOH | carbon | 4-hydroxybutyrate | ||
| Gondo acid | Eicos-11-enoic acid |

|
H 3 C- (CH 2 ) 7 -CH = CH- (CH 2 ) 9 -COOH | carbon | A monounsaturated fatty acid | |||
| Glucaric acid | (2 S , 3 S , 4 S , 5 R ) -2,3,4,5-tetrahydroxyhexane-1,6-diacid |

|
C 6 H 10 O 8 | carbon | Glucarate | A dicarboxylic acid | ||
| Gluconic acid | 2,3,4,5,6-pentahydroxyhexanoic acid |

|
|
C 6 H 12 O 7 | carbon | Gluconates | ||
| Glutaric acid | Pentanedioic acid |

|
|
HOOC- (CH 2 ) 3 -COOH | carbon | 4.32; 5.42 | Glutarate | A dicarboxylic acid |
| Glycolic acid | Hydroxyethanoic acid |

|

|
HIGH 2 -COOH | carbon | 3.83 | Glycolates | |
| Glyoxalic acid | Ethanal acid |
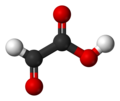
|

|
O = CH-COOH | carbon | 3.18; 3.32 | Glyoxylates | |
| uric acid | 2,6,8-trihydroxypurine |

|
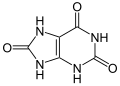
|
C 5 H 4 N 4 O 3 | Carbon , nitrogen | 5.75 | Urates | |
| Hexachloroiridic acid | Dihydrogen hexachloriridate | H 2 [IrCl 6 ] | Iridium , chlorine | Hexachloroiridate | ||||
| Hexachloro-osmic acid | Dihydrogen hexachlorosmat | H 2 [OsCl 6 ] | Iridium , chlorine | Hexachloro-osmates | ||||
| Hexachloroplatinic acid | Dihydrogen hexachloroplatinate | H 2 [PtCl 6 ] | Platinum , chlorine | Hexachloroplatinate | ||||
| Hexafluoroantimonic acid | H [SbF 6 ] | Antimony , fluorine | ||||||
| Hexafluorotitanic acid | Dihydrogen hexafluorotitanate |
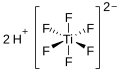
|
H 2 [TiF 6 ] | Titanium , fluorine | Hexafluorotitanates | |||
| Hexafluorozirconic acid | Dihydrogen hexafluorozirconate | H 2 [ZrF 6 ] | Zirconium , fluorine | Hexafluorozirconate | ||||
| Hippuric acid | - |

|
C 9 H 9 NO 3 | Carbon , nitrogen | ||||
| Hypochlorous acid | Hydrogen hypochlorite |

|

|
HClO | chlorine | 7.54 | Hypochlorites | |
| Hypodiphosphonic acid | Tetrahydrogen hypodiphosphonate | H 4 P 2 O 4 | phosphorus | Hypodiphosphonates | ||||
| Hypodiphosphoric acid | Tetrahydrogen hypodiphosphate | H 4 P 2 O 6 | phosphorus | Hypodiphosphates | ||||
| Hypo-nitrous acid | Dihydrogen hyponitrite |
|
|
H 2 N 2 O 2 | nitrogen | 7.21; 11.54 | Hyponitrites | |
| Ibotenic acid | α-amino-3-hydroxy-5-isoxazole acetic acid |

|

|
C 5 H 6 N 2 O 4 | Carbon , nitrogen | A mushroom poison that is contained in the toadstool , among other things | ||
| Indole-3-acetic acid | 1 H -indole-3-ethanoic acid |

|

|
C 10 H 9 NO 2 | Carbon , nitrogen | 4.75 | ||
| Iodic acid | Hydrogen iodate |

|

|
HIO 3 | Iodine | 0.804 | Iodates | |
| Hydriodic acid | Hydrogen iodide | HI (aq) | Iodine | −10.0 | Iodides | Aqueous solution of hydrogen iodide . A super acid . | ||
| Isocitric acid | 3-carboxy-2-hydroxypentane-1,5-diacid |

|

|
C 6 H 8 O 7 | carbon | Isocitrate | A tricarboxylic acid | |
| Isocyanic acid | Hydrogen isocyanate |
|

|
HNCO | Carbon , nitrogen | 3.92 | Cyanates | |
| Isophthalic acid | 1,3-benzene dicarboxylic acid |
|
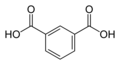
|
C 8 H 6 O 4 | carbon | 3.62; 4.60 | Isophthalates | A dicarboxylic acid |
| α-ketoglutaric acid | 2-oxopentanedioic acid |

|

|
C 5 H 6 O 5 | carbon | α-ketoglutarates | A dicarboxylic acid and a keto acid | |
| Silica | H 4 SiO 4 | Silicon | Silicates | There are several silicas . | ||||
| Pop acid | Oxidoazaniumylidynemethane |
|
|
HCNO | Carbon , nitrogen | Fulminates | ||
| Aqua regia | - | Mixture of 3 parts hydrochloric acid and 1 part nitric acid | ||||||
| carbonic acid | Dihydrogen carbonate |

|
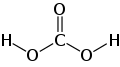
|
H 2 CO 3 | carbon | 3.6; 10.3 | Carbonates | Formed by the reaction of carbon dioxide with water |
| Lauric acid | Dodecanoic acid |
|
|
H 3 C- (CH 2 ) 10 -COOH | carbon | 5.3 | Laurate | |
| Lignoceric acid | Tetracosanoic acid |
|
|
H 3 C- (CH 2 ) 22 -COOH | carbon | Lignocerate | ||
| α-linolenic acid | (all- cis ) -Octadeca-9,12,15-trienoic acid |
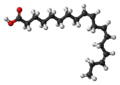
|
|
C 18 H 30 O 2 | carbon | A triunsaturated fatty acid | ||
| Linoleic acid | ( cis , cis ) -Octadeca-9,12-dienoic acid |
|
|
C 18 H 32 O 2 | carbon | 4.77 | Linoleate | |
| Magical acid | - | Mixture of fluorosulfonic acid and antimony (V) fluoride | ||||||
| Maleic acid | (2 Z ) -But-2-enedioic acid |

|
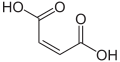
|
HOOC-CH = CH-COOH | carbon | 1.9; 6.5 | Maleates | A monounsaturated dicarboxylic acid |
| Malonic acid | Propanedioic acid |

|
|
HOOC-CH 2 -COOH | carbon | 2.83; 5.69 | Malonates | A dicarboxylic acid , which in beet is |
| Mandelic acid | 2-hydroxy-2-phenylethanoic acid |

|
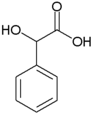
|
C 8 H 8 O 3 | carbon | 3.37 | ||
| Manganic acid | Hydrogen manganate | H 2 MnO 4 | manganese | Manganates | ||||
| Margaric acid | Heptadecanoic acid |
|
|
H 3 C- (CH 2 ) 15 -COOH | carbon | Heptadecanoate | A saturated fatty acid and alkanoic acid | |
| Melissic acid | Triacontanoic acid |
|
C 29 H 59 COOH | carbon | A saturated fatty acid and alkanoic acid | |||
| Metasilicic acid | - | H 2 SiO 3 | Silicon | Metasilicates | One of several silicas | |||
| Methanesulfonic acid | - |

|

|
CH 3 S-O 3 H | Carbon , sulfur | −1.9 | Mesilates | |
| Lactic acid | 2-hydroxypropanoic acid |
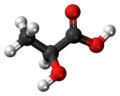
|

|
H 3 C-CH (OH) -COOH | carbon | 3.90 | Lactates | Due to their optical activity, forms the enantiomers L -lactic acid and D -lactic acid |
| Molybdic acid | Dihydrogen molybdate |
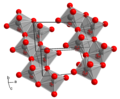
|
H 2 MoO 4 | molybdenum | 3.7; 3.9 | Molybdates | ||
| Montanic acid | Octacosanoic acid |
|
|
C 27 H 55 COOH | carbon | Montanoate | A saturated fatty acid and alkanoic acid | |
| Myristic acid | Tetradecanoic acid |
|
|
H 3 C- (CH 2 ) 12 -COOH | carbon | Myristates | A saturated fatty acid and alkanoic acid | |
| N- acetyl neuraminic acid | - |

|
C 10 H 19 NO 9 | Carbon , nitrogen | ||||
| Nervonic acid | Delta 15 cis tetracosenoic acid |
|
H 3 C- (CH 2 ) 7 -CH = CH- (CH 2 ) 13 -COOH | carbon | A monounsaturated fatty acid | |||
| Oleic acid | (9 Z ) -Octadec-9-enoic acid |
|
|
H 3 C- (CH 2 ) 7 -CH = CH- (CH 2 ) 7 -COOH | carbon | Oleates | A monounsaturated fatty acid | |
| Enanthic acid | Heptanoic acid |
|
|
H 3 C- (CH 2 ) 5 -COOH | carbon | 4.89 | Heptanoates | A saturated fatty acid and alkanoic acid |
| Orthodilicic acid | Hexahydrogen disilicate |

|
H 6 Si 2 O 7 | Silicon | Orthodisilicates | One of several silicas | ||
| Orthosilicic acid | Tetrahydrogensilicate | H 4 SiO 4 | Silicon | Orthosilicates | One of several silicas | |||
| Oxaloacetic acid | 2-oxo-butanedioic acid |

|

|
HOOC-CH 2 -CO-COOH | carbon | Oxaloacetates | A dicarboxylic acid | |
| Oxalic acid | Ethanedioic acid |
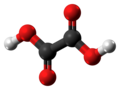
|

|
HOOC-COOH | carbon | 1.23; 4.19 | Oxalates | The simplest dicarboxylic acid |
| Palmitic acid | Hexadecanoic acid |
|
|
H 3 C- (CH 2 ) 14 -COOH | carbon | 4.75 | Palmitates | A saturated fatty acid and alkanoic acid |
| Palmitoleic acid | (9 Z ) -hexadec-9-n acid |
|
|
H 3 C- (CH 2 ) 5 -CH = CH- (CH 2 ) 7 -COOH | carbon | A monounsaturated fatty acid | ||
| Pelargonic acid | Nonanoic acid |
|
H 3 C- (CH 2 ) 7 -COOH | carbon | 4.96 | Pelargonates | A saturated fatty acid and alkanoic acid | |
| Perchloric acid | Hydrogen perchlorate |

|

|
HClO 4 | chlorine | −10.0 | Perchlorates | A super acid |
| Permanganic acid | Dihydrogen permanganate | H 2 MnO 4 | manganese | Permanganate | ||||
| Peroxodisulfuric acid | Dihydrogen dipersulfate |

|
|
H 2 S 2 O 8 | sulfur | Peroxodisulfates | ||
| Peroxodiphosphoric acid | Tetrahydrogen peroxodiphosphate |
|
H 4 P 2 O 8 | phosphorus | Peroxodiphosphates | |||
| Peroxophosphoric acid | Trihydrogen peroxophosphate | H 3 PO 5 | phosphorus | Peroxophosphates | ||||
| Peroxo nitric acid | Hydrogen peritrate |
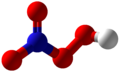
|

|
ENT 4 | nitrogen | Pernitrate | ||
| Perrhenic acid | Hydrogen perrhenate |

|
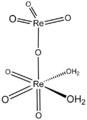
|
HReO 4 | rhenium | −1.25 | Perrhenate | |
| Pertechnetic acid | Hydrogen pertechnetate |
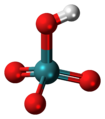
|
HTcO 4 | Technetium | Pertechnetates | |||
| Perxenonic acid | Tetrahydrogen perxenate |
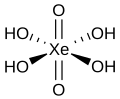
|
H 4 XeO 6 | xenon | Perxenate | Contains the noble gas xenon | ||
| Phenylacetic acid | 1-benzene ethanoic acid |
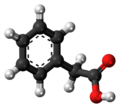
|

|
C 8 H 8 O 2 | carbon | 4.28 | ||
| Phosphinic acid | Trihydrogen phosphinate |

|

|
H 3 PO 2 | phosphorus | 2.0; 2.23 | Phosphinates | |
| Phosphonic acid | Trihydrogen phosphonate |

|

|
H 3 PO 3 | phosphorus | 2.0; 6.59 | Phosphonates | |
| phosphoric acid | Trihydrogen phosphate |
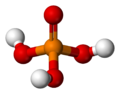
|

|
H 3 PO 4 | phosphorus | 2.16; 7.21; 12.32 | Phosphates | Is using phosphorus pentoxide produced |
| Phthalic acid | 1,2-benzene dicarboxylic acid |

|
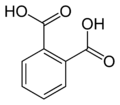
|
C 8 H 6 O 4 | carbon | 2.95; 5.41 | Phthalates | A dicarboxylic acid |
| Picric acid | 2,4,6-trinitrophenol |

|

|
C 6 H 3 N 3 O 7 | Carbon , nitrogen | 0.29 | Picrates | |
| Pimelic acid | Heptanedioic acid |

|
|
C 7 H 12 O 4 | carbon | 4.47; 5.52 | Pimelates | |
| Platinic acid | Dihydrogen hexahydroxoplatinate (VI) | H 2 Pt (OH) 6 | platinum | Platinates | ||||
| Propionic acid | Propanoic acid |

|

|
H 3 C-CH 2 -COOH | carbon | 4.87 | Propionate | An alkanoic acid |
| Squaric acid | 3,4-dihydroxycyclobut-3-ene-1,2-dione |

|
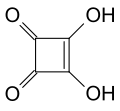
|
C 4 H 2 O 4 | carbon | 1.5; 3.4 | Quadratate | A derivative of cyclobutene . An organic acid without a carboxy group . |
| Ricinoleic acid | 12-Hydroxy- (9 Z ) -octadec-9-enoic acid |

|
C 18 H 34 O 3 | carbon | ||||
| Salicylic acid | 2-hydroxybenzoic acid |

|

|
C 7 H 6 O 3 | carbon | 2.75; 12.38 | Salicylates | Is very similar to benzoic acid |
| nitric acid | Hydrogen nitrate |

|
|
ENT 3 | nitrogen | −1.37 | Nitrates | Mesomerism occurs in this connection . |
| Nitrous acid | Hydrogen nitrite |
 |

|
ENT 2 | nitrogen | 3.29 | Nitrites | When using nitrogen dioxide produced |
| hydrochloric acid | Hydrochloric acid / hydrogen chloride |

|
HCl (aq) | chlorine | −5.9 | Chlorides | Aqueous solution of hydrogen chloride . A super acid . | |
| sulfuric acid | Dihydrogen sulfate |

|

|
H 2 SO 4 | sulfur | −3.0; 1.9 | Sulfates | When using sulfur trioxide produced |
| Hydrogen sulfide | Dihydrogen sulfide |

|

|
H 2 S | sulfur | 7.00; 12.92 | Sulphides | |
| sulphurous acid | Dihydrogen sulfite |

|

|
H 2 SO 3 | sulfur | 1.81; 6.99 | Sulfites | Formed by the reaction of sulfur dioxide with water |
| Shikimic acid | 3,4,5-trihydroxy-1-cyclohexenecarboxylic acid |

|

|
C 7 H 10 O 5 | carbon | 4.15 | Shikimate | |
| Sorbic acid | (2 U , 4 U ) -2,4-hexadienoic acid |

|
|
C 6 H 8 O 2 | carbon | 4.76 | Sorbates | A doubly unsaturated carboxylic acid |
| Stearic acid | Octadecanoic acid |
|
|
H 3 C- (CH 2 ) 16 -COOH | carbon | Stearates | A saturated fatty acid and alkanoic acid | |
| Hydrazoic acid | Hydrogen azide |

|
|
HN 3 | nitrogen | 4.6; 7.9 | Azides | Made with hydrazine . Mesomerism occurs in this connection . |
| Styphnic acid | 2,4,6-trinitro-1,3-hydroxybenzene |

|
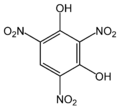
|
C 6 H 3 N 3 O 8 | Carbon , nitrogen | Styphnate | An organic acid without a carboxy group | |
| Sulfanilic acid | 4-amino-1-benzenesulfonic acid |

|

|
C 6 H 7 NO 3 S | Carbon , nitrogen | 3.23 | An organic acid without a carboxy group | |
| Telluric acid | Hexahydrogen tellurate |
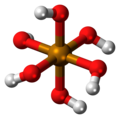
|

|
H 6 TeO 6 | Tellurium | 7.70; 10.95 | Tellurates | |
| Terephthalic acid | Benzene-1,4-dicarboxylic acid |
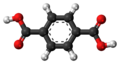
|
|
C 8 H 6 O 4 | nitrogen | 3.54; 4.46 | Terephthalates | A dicarboxylic acid |
| Tetrachloroauric acid | Hydrogen tetrachloroaurate |

|
H [AuCl 4 ] | Gold , chlorine | Tetrachloroaurate | |||
| Tetrahydrofolic acid |
N - [(6 S ) -5,6,7,8-tetrahydropteroyl] -L-glutamic acid |

|

|
C 19 H 23 N 7 O 6 | Carbon , nitrogen | 3.51 | A dicarboxylic acid | |
| Thiosulfuric acid | Dihydrogen thiosulfate |

|

|
H 2 S 2 O 3 | sulfur | 0.6; 1.74 | Thiosulfates | |
| Trichloroacetic acid | Trichloroethanoic acid |

|
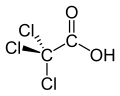
|
Cl 3 C-COOH | Carbon , chlorine | 0.65 | Trichloroacetate | |
| Trifluoromethanesulfonic acid |
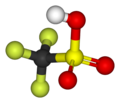
|

|
CF 3 SO 3 H | Carbon , sulfur , fluorine | −20.0 | Triflates | One of the strongest super acids | |
| Trifluoroacetic acid | Trifluoroethanoic acid |

|

|
F 3 C-COOH | Carbon , fluorine | 0.23 | Trifluoroacetate | |
| Trilicic acid | (HO) 3 Si-O-Si (OH) 2 -O-Si (OH) 3 . | Silicon | Trisilicates | One of several silicas | ||||
| Valeric acid | Pentanoic acid |

|

|
H 3 C- (CH 2 ) 3 -COOH | carbon | 4.84 | Valerates | A saturated fatty acid and alkanoic acid |
| Vanadium acid | Hydrogen vanadate | H 3 VO 4 | Vanadium | Vanadates | ||||
| Vulpinic acid | - |

|

|
C 19 H 14 O 5 | carbon | A toxin , which in some lichens contained | ||
| Tartaric acid | 2,3-dihydroxybutanedioic acid |

|
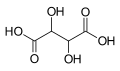
|
HOOC-CH (OH) -CH (OH) -COOH | carbon | 2.98; 4.34 | Tartrates | A dicarboxylic acid |
| Tungstic acid | Dihydrogen tungstate |

|
H 2 WO 4 | tungsten | 3.5; 4.6 | Wolframates | ||
| Xenonic acid | Dihydrogen xenate |

|

|
H 2 XeO 4 | xenon | 10.5 | Xenate | Contains the noble gas xenon |
| Cinnamic acid | 3-phenylpropenoic acid |

|
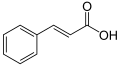
|
C 9 H 8 O 2 | carbon | 4.44 |























































![{\ displaystyle {\ ce {H [Sb (OH) 6]}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/e30797e3df56119b39db7c4c3ae7b5a3ae76a236)





![{\ displaystyle {\ ce {H [AuCl4]}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/9a6aeecf36990cee03025ebad8e30c1302f7b29a)
![{\ displaystyle {\ ce {H2 [IrCl6]}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/f06b5c8864a7c410556eb8241940f35abbfbc917)
![{\ displaystyle {\ ce {H2 [PtCl6]}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/f6db11a0f2bd82fd13760335066370b01133131e)

![{\ displaystyle {\ ce {H2 [OsCl6]}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/68baeca2b2743578e2b42927bf9f707676d5f01e)
![{\ displaystyle {\ ce {H2 [TiF6]}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/213c350639ec33a292ea5a71493f482f1f8ce86e)
![{\ displaystyle {\ ce {H2 [ZrF6]}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/8434b0afaa50f73d2e68793c7c28442e82e5e990)








