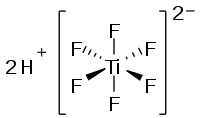
Hexafluorotitanic acid
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Hexafluorotitanic acid | |||||||||||||||
| other names |
Dihydrogen hexafluorotitanate |
|||||||||||||||
| Molecular formula | H 2 [TiF 6 ] | |||||||||||||||
| Brief description |
colorless liquid with a pungent odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 163.88 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
1.675 g cm −3 |
|||||||||||||||
| Melting point |
<0 ° C |
|||||||||||||||
| boiling point |
> 100 ° C (1013 hPa ) |
|||||||||||||||
| Vapor pressure |
23 hPa (20 ° C) |
|||||||||||||||
| solubility |
completely miscible with water |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Hexafluorotitanic acid , H 2 [TiF 6 ], is an inorganic acid which consists of titanium and fluorine . Their salts are called hexafluorotitanates.
Extraction and presentation
Hexafluorotitanic acid can be made by dissolving titanium dioxide in hydrofluoric acid .
Alternatively, hexafluorotitanic acid can be produced by reacting metallic titanium with hydrofluoric acid.
properties
A 60% aqueous solution of hexafluorotitanic acid is a colorless liquid with a pungent odor. It has a pH value of <1.
use
Hexafluorotitanic acid can be used for the surface treatment of metals.
safety instructions
The compound is toxic if swallowed or inhaled . Serious burns are consequences of skin contact with the acid. It causes severe damage to the eyes.
Compounds such as bases , cyanides and glass as well as metals should not come into contact with the acid.
Individual evidence
- ↑ a b c d e f g data sheet hexafluorotitanic acid solution from Sigma-Aldrich , accessed on March 6, 2019 ( PDF ).
- ↑ a b c d Entry on dihydrogen hexafluorotitanate (2-), 60% aqueous solution in the GESTIS substance database of the IFA , accessed on January 8, 2020(JavaScript required) .
- ↑ Gmelin Institute for Inorganic Chemistry and Limits: Titan . Springer-Verlag, 2013, ISBN 978-3-662-13217-3 , pp. 289 ( limited preview in Google Book search).
- ↑ a b Cuie Wen: Surface Coating and Modification of Metallic Biomaterials . Woodhead Publishing, 2015, ISBN 978-1-78242-316-4 , pp. 163 ( limited preview in Google Book search).
- ↑ George E. Totten, D. Scott MacKenzie: Handbook of Aluminum Volume 2: Alloy Production and Materials Manufacturing . CRC Press, 2003, ISBN 978-0-203-91260-7 , pp. 494 ( limited preview in Google Book search).


![{\ displaystyle \ mathrm {TiO_ {2} +6 \ HF \ longrightarrow \ H_ {2} [TiF_ {6}] + 2 \ H_ {2} O}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/b1a9717f49648dbf6e5cd11f8838635bb9aadcb4)
![{\ displaystyle \ mathrm {Ti + 6 \ HF \ longrightarrow \ H_ {2} [TiF_ {6}] + 2 \ H_ {2}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/13a38ee71c4f998c1b877d7b42e976df6b7cea8e)