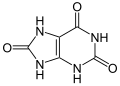
uric acid
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||||||||
| Keto form of uric acid | ||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | uric acid | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 5 H 4 N 4 O 3 | |||||||||||||||||||||
| Brief description |
odorless light beige solid |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 168.11 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| density |
1.89 g cm −3 |
|||||||||||||||||||||
| Melting point |
> 300 ° C |
|||||||||||||||||||||
| pK s value |
5.75 |
|||||||||||||||||||||
| solubility |
little in water |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Uric acid (clinical abbreviation: "HS", but not to be confused with urea ) is the end product of nucleic acid breakdown (here: breakdown of purine bases ) in many animal species, such as reptiles, birds, monkeys and humans. In reptiles and birds, amino acids are also broken down into uric acid. The salts of uric acid are called urates .
structure
A lactam-lactim tautomerism can be formulated for uric acid :
The keto form is preferred over the heteroaromatic 2,6,8-trihydroxypurine.
presentation
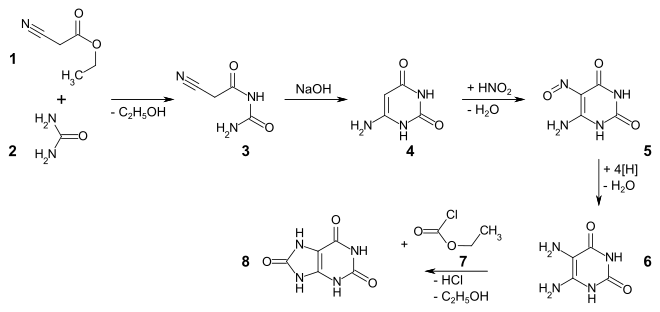
Uric acid can be represented in a grape synthesis . The condensation of ethyl cyanoacetate 1 with urea 2 produces cyanoacetylurea 3 , which can be cyclized to 6-aminouracil 4 under basic conditions . Via the nitroso compound 5 is 5,6-diaminouracil 6 accessible, which with ethyl chloroformate 7 to uric acid 8 responds.
properties
Uric acid forms white, odorless crystals that melt from 300 ° C and occurs in two tautomeric forms (see structural formula). As a weak acid, uric acid is poorly soluble in a protonated state (for example in water ), but it is readily soluble in basic media. The alkali salts (especially the lithium salt ) also have better water solubility.
Biological importance
physiology

In the organism of hominids , i.e. humans , chimpanzees , gorillas and orangutans , uric acid is produced as a breakdown product of the purine bases and is therefore the end product of purine metabolism. It is produced from hypoxanthine or xanthine by the enzyme xanthine oxidase . Uric acid is the final breakdown product of the purine nucleotides and about 75% is excreted renally , i.e. via the kidneys. In addition, there is also elimination via saliva , sweat or intestinal secretion, i.e. via the intestine. The daily excretion is up to 1 g.
In other mammals , uric acid is converted to allantoin by the uricase enzyme .
Although hominids cannot break down uric acid any further, they have an effective reabsorption system in the kidneys in the form of the uric acid / anion exchanger URAT1 . Because of this, they have five to ten times higher serum uric acid levels than other mammals. One possible reason for the high levels of uric acid in the blood could be its antioxidant properties.
The predominant excretion of excess nitrogen via uric acid, which only affects other animals, is called uricotelia .
In the marine polychaete Platynereis dumerilii , uric acid occurs as a pheromone , which is released into the water by the females when the animals mate. There it triggers the release of sperm in the male.
Physical chemistry
Uric acid has different manifestations, reduced and oxidized. Consequently, it depends on the environment around the uric acid which redox state is present. Then it is also decided whether and for how long a bond is entered into with a reaction partner.
Pathophysiology
Under certain conditions, an increased amount of uric acid can occur in the organism. The most common reason is insufficient uric acid excretion from the kidneys. If the solubility product is exceeded, the uric acid can precipitate and be deposited in the urinary tract , in the bloodstream and in bradytrophic tissues. The pH value also plays a role here: while uric acid in the blood is largely dissociated and therefore soluble at a pH of 7.4, it crystallizes easily at a more acidic pH. This is z. B. in urine or in tissues with a low oxygen supply (and thereby increased lactate formation).
This hyperuricemia can result in uroliths (stones in uric acid lithiasis ), gout and uric acid infarctions . The sodium salt of uric acid, sodium urate , plays an important role here because it then settles in the form of crystals (gout) or stones ( kidney stones ).
Certain factors increase uric acid production or the amount of uric acid in the body:
- Nutrition:
- Increased intake of fructose- containing foods (sugary drinks, sweets, sugared breakfast cereals, etc.) is associated with an increased risk of obesity , gout and high blood pressure
- Increased intake of foods rich in purine
-
Diseases :
- Hemoblastosis
- Glycogenoses
- Lesch-Nyhan syndrome
- Idiopathic hypercalcemia
- Hypertriglyceridemia
- Chronic kidney failure (renal failure)
- Acute tumor lysis syndrome
- Side effects of various forms of therapy :
The determination of the uric acid concentration is of great importance in tumor therapy with cytostatics or ionizing radiation . If larger tumor and cell masses are destroyed, the uric acid content in the blood rises rapidly, which can lead to severe kidney damage. The tumor therapy must be controlled by regular monitoring so that critical uric acid levels are not reached.
Ethanol inhibits uric acid excretion.
In a large epidemiological study, elevated uric acid levels in the normal population were a moderate risk factor of developing chronic kidney disease in the further course .
Metabolic syndrome
The founder of pathology , Giovanni Battista Morgagni (1682–1771; professor in Padua), recognized the connection between obesity , diabetes , high blood pressure and gout as early as the 18th century . In the "first description" of the Metabolic Syndrome (MetS) by the Swede E. Kylin in 1923, hyperuricemia is mentioned in addition to the increase in body weight, blood lipids and blood sugar . In the currently valid definitions z. B. the International Diabetes Foundation (IDF) is missing hyperuricemia. However, an increasing number of scientists are including them again in their definition of the MetS.
Detection reactions
The uric acid content can be measured in the enzyme test by photometry using urate oxidase and an absorption in the range of 290 nm.
Another common proof is the evaporation of the uric acid with concentrated nitric acid and the addition of ammonia solution in the murexide sample.
Web links
- Wissenschaft.de: How uric acid can prevent paralysis
- Nutrition.de: uric acid content of foods (table; PDF, 77 kB)
Individual evidence
- ↑ Entry on URIC ACID in the CosIng database of the EU Commission, accessed on March 30, 2020.
- ↑ a b c d e data sheet uric acid (PDF) from Carl Roth , accessed on December 18, 2012.
- ↑ Shmuel Yannai: Dictionary of Food Compounds with CD-ROM, Second Edition . CRC Press, 2012, ISBN 978-1-4200-8352-1 , pp. 2025 ( limited preview in Google Book Search).
- ↑ Eberhard Breitmaier, Günther Jung: Organic chemistry . Basics, substance classes, reactions, concepts, molecular structure. 5th edition. Georg Thieme Verlag, Stuttgart 2005, ISBN 3-13-541505-8 , p. 642 .
- ↑ Hans Beyer, Wolfgang Walter: Textbook of organic chemistry . 18th edition. S. Hirzel Verlag, Stuttgart 1978, ISBN 3-7776-0342-2 , p. 703 .
- ↑ Deutsches Ärzteblatt: Uric acid slows down the progression of Parkinson's ( Memento from August 13, 2014 in the Internet Archive ).
- ↑ Erich Zeeck et al .: Uric acid: The sperm-release pheromone of the marine polychaete Platynereis dumerilii . In: J Chem Ecol . No. 24 , 1998, pp. 13-22 , doi : 10.1023 / A: 1022328610423 .
- ↑ Ursula Gresser : Diagnosis and Therapy of Gout , in: Dtsch Arztebl 2003, 100 (44): A-2862 / B-2379 / C-2235.
- ^ Daniel E. Weiner et al .: Uric Acid and Incident Kidney Disease in the Community . In: J Am Soc Nephrol . No. 19 , 2008, p. 1204-1211 , doi : 10.1681 / ASN.2007101075 .