Ethyl cyanoacetate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
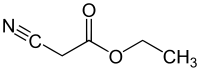
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Ethyl cyanoacetate | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 5 H 7 NO 2 | |||||||||||||||
| Brief description |
colorless clear liquid with a faint aromatic odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 113.116 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
1.06 g cm −3 (20 ° C) |
|||||||||||||||
| Melting point |
−22 ° C |
|||||||||||||||
| boiling point |
209 ° C (1013 hPa) |
|||||||||||||||
| Vapor pressure |
<10 Pa |
|||||||||||||||
| solubility |
|
|||||||||||||||
| Refractive index |
1.4175 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Ethyl cyanoacetate is a chemical compound that belongs to the carboxylic acid esters and nitriles and does not occur in nature. The clear, colorless to slightly yellowish liquid has a pleasant odor and has three reactive centers in the molecule with an ester, a cyano and a CH-acidic methylene group .
Extraction and presentation
Ethyl cyanoacetate can be produced in several ways:
- by esterification of cyanoacetic acid with ethanol in the presence of strong mineral acids (e.g. concentrated sulfuric acid ).
- by Kolbe nitrile synthesis from ethyl chloroacetate with sodium cyanide .
- by Kolbe nitrile synthesis from sodium chloroacetate and sodium cyanide, acidification to form cyanoacetic acid and esterification with ethanol according to 1.
- by reacting the sodium salt of cyanoacetic acid with ethyl bromide in an aqueous-organic two-phase system in the presence of a phase transfer catalyst .
- by oxidation of 3-ethoxypropionitrile with oxygen under pressure in the presence of the catalyst cobalt (II) acetate tetrahydrate and a radical generator, e.g. B. N -hydroxyphthalimide .
properties
Physical Properties
Ethyl cyanoacetate is a colorless liquid that boils at 209 ° C under normal pressure. According to Antoine, the vapor pressure function results from log 10 (P) = A− (B / (T + C)) (P in bar, T in K) with A = 7.46724, B = 3693.663 and C = 16.138 im Temperature range from 341 to 479 K. Two polymorphic forms can occur in the solid phase . The crystal form II is below −111 ° C. Above this temperature there is crystal form I, which melts at −22 ° C. The heat capacity at 25 ° C is 220.22 J · K −1 · mol −1 .
Chemical properties
With its three different reactive centers, ethyl cyanoacetate is a versatile synthetic building block for a large number of functional and pharmacologically active substances. In terms of its reactivity, it is similar to diethyl malonate , which is obtained from ethyl cyanoacetate by reaction with ethanol in the presence of strong acids (danger of the formation of diethyl malonate during the esterification of cyanoacetic acid, see). Ethyl cyanoacetate is therefore an important starting material for condensation reactions in the sense of the Knoevenagel condensation and the Michael addition . Due to its reactivity and multifunctionality, ethyl cyanoacetate forms the dimeric diethyl 3-amino-2-cyano-2-pentenedioate when heated in the presence of sodium ethoxide.
use
Due to its functionality, ethyl cyanoacetate reacts:
- on the nitrile group: hydrogenation leads to the β-amino acid β-alanine .
- on the ester group: reaction with ammonia leads to cyanoacetamide , which can be converted to malononitrile by dehydration with PCl 5 or POCl 3 .
- on the CH-acidic methylene group: z. B. Reaction with formaldehyde to form 2-cyanoacrylic acid ethyl ester , which is used as a superglue .
Ethyl cyanoacetate is a building block for the construction of heterocycles which z. B. find use as drugs.
The synthesis of allopurinol for the treatment of chronic gout starts from diethyl cyanoacetate and ethyl orthoformate, the condensation product of which is cyclized with hydrazine to give a substituted pyrazole and then with formamide to give allopurinol, a substituted pyrazolopyrimidine.
The purine derivatives theophylline , caffeine and uric acid are synthetically accessible from ethyl cyanoacetate and N , N '-dimethylurea .
The pteridine derivative folic acid is a vitamin assigned to the B complex and is produced from ethyl cyanoacetate and guanidine via a multi-stage convergent synthesis.
The pyrrole derivative ethosuximide is used to treat epilepsy and is obtained from ethyl cyanoacetate and 2-butanone in a multi-stage synthesis.
The pyrimidine derivative trimethoprim is used as a co- trimoxazole in fixed combination with sulfamethoxazole as a bacteriostatic and is synthesized from ethyl cyanoacetate and 3,4,5-trimethoxybenzaldehyde or benzyl chloride.
In addition, a variety of other functional heterocycles, such as. B. 3-substituted coumarin derivatives from ethyl cyanoacetate accessible in good yields.
Another important acyclic pharmaceutical agent derived from ethyl cyanoacetate is the anticonvulsant valproic acid .
safety instructions
Ethyl cyanoacetate is not a dangerous product in the sense of the CLP regulation , but its vapors or aerosols can cause eye irritation. Vapors / aerosols should not be inhaled and the liquid should not enter drains. Ethyl cyanoacetate is flammable and can form carbon monoxide, carbon dioxide, nitrogen oxides and hydrogen cyanide when burned. Contact with strong bases or acids as well as strong oxidizing and reducing agents can lead to violent reactions.
literature
- Mary Eagleson: Concise encyclopedia chemistry , Walter de Gruyter, Berlin - New York 1994, ISBN 3-11-011451-8 .
- A. Kleemann , J. Engel: Pharmaceutical active ingredients , 2nd edition, Georg Thieme, Stuttgart - New York 1982, ISBN 3-13-558402-X .
- Beyer-Walter: Textbook of Organic Chemistry , 24th edition, S. Hirzel, Stuttgart - Leipzig 2004.
Individual evidence
- ↑ a b c d e f Entry for CAS no. 105-56-6 in the GESTIS substance database of the IFA , accessed on March 3, 2011(JavaScript required) .
- ↑ a b c d Data sheet ethyl cyanoacetate (PDF) from Merck , accessed on June 13, 2011.
- ↑ Entry on cyano (o) acetic acid ester. In: Römpp Online . Georg Thieme Verlag, accessed on March 5, 2011.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-240.
- ↑ a b Entry on Ethyl cyanoacetate in the ChemIDplus database of the United States National Library of Medicine (NLM), accessed on July 31, 2018.
- ↑ a b J. KH Inglis: Ethyl Cyanoacetate In: Organic Syntheses . 8, 1928, p. 74, doi : 10.15227 / orgsyn.008.0074 ; Coll. Vol. 1, 1941, p. 254 ( PDF ).
- ↑ European patent application EP-A 1028105, priority: February 9, 1999, applicant: Lonza AG.
- ↑ European patent specification EP 1208081, priority: August 30, 1999, applicant: Lonza AG.
- ↑ Stull, DR: Vapor Pressure of Pure Substances Organic Compounds in Ind. Eng. Chem. 39 (1947) 517-540, doi : 10.1021 / ie50448a022 .
- ↑ a b c Khodzhaeva, MG; Bugakov, Yu.V .; Ismailov, TS: Heat capacity and thermodynamic functions of ethyl cyanoacetate in Khim. Farm. Zhur. 21 (1987) 760-762.
- ↑ VA Dorokhov et al., Russ. Chem. Bulletin, Vol. 41 (2), 287-291, 1992 .
- ^ Mary Eagleson: Concise encyclopedia chemistry , Walter de Gruyter, Berlin - New York 1994, ISBN 3-11-011451-8 .
- ↑ Axel Kleemann , Jürgen Engel: "Pharmaceutical Active Ingredients", 2nd edition, Georg Thieme, Stuttgart - New York 1982, ISBN 3-13-558402-X .
- ↑ Beyer-Walter: "Textbook of Organic Chemistry", 24th edition, S. Hirzel, Stuttgart - Leipzig 2004.
- ↑ AA Avetisyan et al., Chem. Heterocycl. Compounds, Vol. 15 (9), 959-960, 1980 .
