Ethyl chloroacetate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
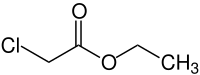
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Ethyl chloroacetate | |||||||||||||||
| other names |
Ethyl chloroacetate |
|||||||||||||||
| Molecular formula | C 4 H 7 ClO 2 | |||||||||||||||
| Brief description |
Flammable, colorless liquid with a pungent odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 122.55 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
1.16 g cm −3 |
|||||||||||||||
| Melting point |
−26 ° C |
|||||||||||||||
| boiling point |
145 ° C |
|||||||||||||||
| Vapor pressure |
4.8 hPa (20 ° C) |
|||||||||||||||
| solubility |
|
|||||||||||||||
| Refractive index |
1.421 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data |
|
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Ethyl chloroacetate is a chemical compound from the group of substituted carboxylic acid esters and organic chlorine compounds .
presentation
Ethyl chloroacetate can be produced by a condensation reaction of chloroacetic acid and ethanol .
A synthesis from dichlorovinyl ethyl ether is also described.
properties
Ethyl chloroacetate is a flammable, colorless, tear-irritating liquid with a pungent odor, which is sparingly soluble in water. It has a dynamic viscosity of 1.27 mPa · s at 20 ° C.
use
Ethyl chloroacetate is used as a solvent and in organic syntheses.
safety instructions
The vapors of ethyl chloroacetate can form an explosive mixture with air ( flash point 54 ° C, ignition temperature 452 ° C, lower explosion limit 2.6% by volume).
Individual evidence
- ↑ a b c d e f g h i j k l m n Entry on ethyl chloroacetate in the GESTIS substance database of the IFA , accessed on December 22, 2019(JavaScript required) .
- ↑ Data sheet ethyl chloroacetate from Acros, accessed on October 27, 2010.
- ^ IUPAC: Critical compilation of scales of solvent parameters . (PDF; 494 kB).
- ↑ Entry on Ethyl chloroacetate in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ LJ Simon, G. Chavanne: "Une preparation nouvelle de l'acide monochloracétique" in CR Hebd. 1923 , 176 , p. 309 ( full text ).
- ↑ Data sheet ethyl chloroacetate (PDF) from Merck , accessed on October 27, 2010.
- ↑ Entry on Ethyl chloroacetate in the Hazardous Substances Data Bank , accessed July 29, 2012.




