N -hydroxyphthalimide
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | N-hydroxyphthalimide | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 8 H 5 NO 3 | |||||||||||||||
| Brief description |
crystalline white or yellow solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 163.13 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density | ||||||||||||||||
| Melting point |
|
|||||||||||||||
| pK s value |
7th |
|||||||||||||||
| solubility |
soluble in water (50.5 g l −1 at 25 ° C) and in acetic acid , acetonitrile and ethyl acetate |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
N -hydroxyphthalimide (NHPI) is the N -hydroxy derivative of phthalimide . The connection is u. a. as a catalyst for oxidation reactions, especially for the selective oxidation of u. a. Alkanes are used to form alcohols with molecular oxygen under mild conditions.
Occurrence and representation
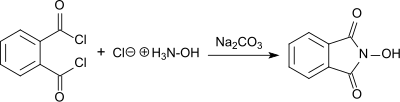
The synthesis of N -hydroxyphthalimide (known as "phthalylhydroxylamine") from phthaloyl chloride and hydroxylamine hydrochloride in the presence of sodium carbonate in aqueous solution was first reported in 1880 by L. Cohn.
The red sodium salt is formed in a basic solution, from which the white form of the NHPI precipitates in 55% yield by acidification.
The reaction of diethyl phthalate with hydroxylamine hydrochloride in the presence of sodium ethoxide also leads to N -hydroxyphthalimide.
The reaction of phthalic anhydride with hydroxylamine hydrochloride and Na 2 CO 3 in water at 95 ° C gives N- hydroxyphthalimide as pale yellowish needles in 95% crude yield, from which almost colorless NHPI is obtained in 76% pure yield by recrystallization from water.
Microwave irradiation of phthalic anhydride and hydroxylamine hydrochloride in pyridine produces NHPI in 81% yield.
Even without adding a base, phthalic anhydride and hydroxylamine phosphate react when heated to 130 ° C. to form N- hydroxyphthalimide in 86% yield .
properties
N -hydroxyphthalimide is a colorless to yellow, odorless crystalline powder that is soluble in water and organic solvents such as acetic acid, ethyl acetate and acetonitrile. The compound exists in two different colored monoclinic crystal forms. In the colorless white form, the N-OH group is rotated by approx. 1.19 ° from the molecular plane, in the yellow form only by approx. 0.06 °.
The color formation during the synthesis of the NHPI depends on the type of solvent used; the color transition from white to yellow is irreversible. With alkali and heavy metals, ammonia and amines, NHPI forms strongly colored, mostly yellow or red salts. The hydrolysis of NHPI by adding strong alkalis produces phthalic acid monohydroxamic acid.
NHPI ethers, on the other hand, are colorless and yield O -alkyl hydroxylamines on alkaline hydrolysis or cleavage using hydrazine hydrate .
The molecular structure between phthalic anhydride monooxime (“phthaloxime”) (I), 2,3-benzoxazine-1,4-, which has been uncertain and controversial since the first description of N -hydroxyphthalimide as "phthalylhydroxylamine" in 1880 until the mid-1950s dione (II) and N -hydroxyphthalimide (III)
It was possible to decide in favor of N -hydroxphthalimide (III) by displaying and analyzing reaction products.
Applications
With N -hydroxyphthalimide, as with N -hydroxysuccinimide (HOSu), with carboxylic acids and a carbodiimide , such as. B. Dicyclohexylcarbodiimid , form so-called active esters with elimination of water ,
which have not found widespread use in peptide synthesis compared to the HOSu esters because of their higher water solubility and reactivity.
Esters of N -hydroxyphthalimide with activated sulfonic acids , such as. As trifluoromethanesulfonic anhydride or p -Toluolsulfonsäurechlorid are as so-called Photo acids ( English photoacids ) which, when used in UV irradiation eliminate protons.
The protons generated are used for the targeted local degradation of acid-sensitive photoresists .
N -hydroxyphthalimide can be converted with vinyl acetate in the presence of palladium (II) acetate with 75% yield into N- vinyloxyphthalimide, which is quantitatively hydrogenated to N -ethoxyphthalimide and, by cleavage with hydroxylamine sulphate with 83% yield, O -ethylhydroxylamine ( Ethoxyamine).
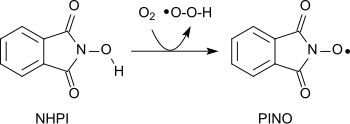
A multitude of different functions in organic molecules can be carried out with the nitroxide radical (phthalimide- N- oxyl, PINO), which is formed by abstraction of a hydrogen atom from NHPI - similar to the piperidine derivative 2,2,6,6-tetramethylpiperidinyloxyl (TEMPO) - oxidized under mild conditions.
Thus, with molecular oxygen, alkanes can be oxidized to alcohols, secondary alcohols to ketones, acetals to esters, or alkenes to epoxides.
Amides can be converted into carbonyl compounds with NHPI and cobalt (II) salts under mild conditions.
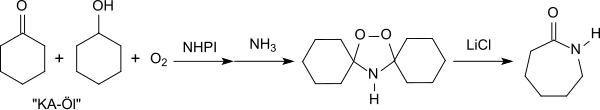
Of technical interest are efficient oxidation reactions of precursors of important basic chemicals, such as. As ε-caprolactam , by using NHPI from the oxidation of cyclohexane resulting so-called KA oil ( "ketone alcohol" oil - a mixture of cyclohexanol and cyclohexanone ) - about Cyclohexanolhydroperoxid, reaction with ammonia to Peroxydicyclohexylamin and rearrangement in the presence catalytic amounts of lithium chloride to ε-caprolactam.
The oxidation of KA oil, catalyzed by NHPI, avoids the formation of the undesired by-product ammonium sulfate that occurs in the conventional ε-caprolactam synthesis - Beckmann rearrangement of cyclohexanone oxime with sulfuric acid .
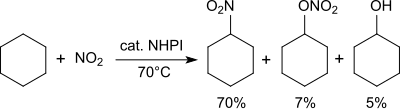
Alkanes are converted into nitroalkanes in the presence of nitrogen dioxide .
For example, cyclohexane is converted into a mixture of nitrocyclohexane (70%), cyclohexyl nitrate (7%) and cyclohexanol (5%) at 70 ° C. with nitrogen dioxide / air .
In addition, uses of N -hydroxyphthalimide as an oxidizing agent in photographic developers and as a charge control agent in toners have been described in the patent literature.
Individual evidence
- ↑ a b c d Data sheet N-Hydroxyphthalimid from Sigma-Aldrich , accessed on June 6, 2017 ( PDF ).
- ↑ Data sheet N-hydroxyphthalimide from AlfaAesar, accessed on August 05, 2016 ( PDF )(JavaScript required) .
- ^ Carl L. Yaws: Thermophysical Properties of Chemicals and Hydrocarbons, 2nd Edition . Elsevier Inc., Oxford, UK 2015, ISBN 978-0-323-28659-6 , pp. 291 .
- ↑ a b Entry on N-hydroxyphthalimide at TCI Europe, accessed on August 5, 2016.
- ↑ Data sheet N-Hydroxyphthalimide from Acros, accessed on August 5, 2016.
- ↑ a b c L. Bauer, SV Miarka: The Chemistry of N-Hydroxyphthalimide . In: J. Am. Chem. Soc. tape 79 , no. 8 , 1957, pp. 1983–1985 , doi : 10.1021 / ja01565a061 .
- ↑ a b A. Porcheddu, G. Giacomelli: The chemistry of hydroxylamines, oximes, and hydroxamic acids, Part 1 . Ed .: Z. Rappaport, JF Lieberman. Wiley, Chichester 2009, ISBN 978-0-470-51261-6 , pp. 224-226 .
- ^ A b C. Gambarotti, C. Punta, F. Recupero, M. Zlotorzynska, G. Sammis: N-Hydrophthalimide . In: e-EROS Encyclopedia of Reagents for Organic Synthesis . 2013, doi : 10.1002 / 047084289X.rn00598.pub2 .
- ↑ a b F. Recupero, C. Punta: Free Radical Functionalization of Organic Compounds Catalyzed by N-hydroxyphthalimide . In: Chem. Rev. Band 107 , no. 9 , 2007, p. 3800-3842 , doi : 10.1021 / cr040170k .
- ↑ L. Melone, C. Punta: Metal-free aerobic oxidations mediated by N-hydroxyphthalimide. A concise review . In: Beilstein J. Org. Chem. Volume 9 , 2013, p. 1296-1310 , doi : 10.3762 / bjoc.9.146 .
- ↑ L. Cohn: Phthalylhydroxylamine: Conversion of phthalic acid into salicylic acid . In: Justus Liebigs Ann. Chem. Band 205 , no. 3 , 1880, p. 295-314 , doi : 10.1002 / jlac.18802050304 .
- ↑ H. Gross, I. Keitel: For the representation of N-hydroxyphthalimide and N-hydroxysuccinimide . In: J. Prakt. Chem. Volume 311 , no. 4 , 1969, p. 692-693 , doi : 10.1002 / prac.19693110424 .
- ↑ K. Sugamoto, Y. Matsushita, Y. Kameda, M. Suzuki, T. Matsui: Microwave-assisted synthesis of N-hydroxyphthalimide . In: Synth. Commun. tape 35 , no. 1 , 2005, p. 67-70 , doi : 10.1081 / SCC-200046498 .
- ↑ Patent EP1085013 : Process for the preparation of cyclic N-hydroxydicarboximides. Registered on August 31, 2000 , published on March 21, 2001 , applicant: Consortium für elektrochemische Industrie GmbH, inventor: E. Fritz-Langhals.
- ^ H. Reichelt, CA Faunce, HH Paradies: Elusive forms and structures of N-hydroxyphthalimide: the colorless and yellow crystal forms of N-hydroxyphthalimide . In: J. Phys. Chem. Band 111 , no. 13 , 2007, p. 2587-2601 , doi : 10.1021 / jp068599y .
- ↑ a b D.E. Ames, TF Gray: N-Hydroxy-imides. Part II. Derivatives of homophthalic and phthalic acid . In: J. Chem. Soc. 1955, p. 3518-3521 , doi : 10.1039 / JR9550003518 .
- ↑ OL Brady, LC Baker, RF Goldstein, S. Harris: LXVIII. - The isomerism of the oximes. Part XXXIII. The oximes of opianic acid and of phthalic anhydride . In: J. Chem. Soc. 1928, p. 529-539 , doi : 10.1039 / JR9280000529 .
- ↑ CD Hurd, CM Buess, L. Bauer: Succino- and phthalo-hydroxamic acids . In: J. Org. Chem. Band 19 , no. 7 , 1954, pp. 1140-1149 , doi : 10.1021 / jo01372a021 .
- ↑ GHL Nefkens, GI Tesser: A novel activated ester in peptide synthesis . In: J. Am. Chem. Soc. tape 83 , no. 5 , 1961, pp. 1263-1263 , doi : 10.1021 / ja01466a068 .
- ↑ M. Bodanszky, A. Bodanszky: The practice of peptide synthesis . Springer, Berlin 1984, ISBN 978-3-642-96837-2 , pp. 124 .
- ↑ Patent EP0919867B1 : Chemically strengthened resist for electron beam lithography. Registered on November 17, 1998 , published on May 21, 2003 , applicant: Infineon Technologies AG, inventor: K. Elian, E. Günther, R. Leuschner.
- ↑ Patent WO1995025090 : Cyclic N-alkenyloxyimides and a method for the preparation of cyclic N-alkenyloxyimides, the corresponding cyclic N-alkoxyimides and O-alkoxyamines. Applied on March 14, 1995 , published on September 21, 1995 , applicant: DSM NV, inventor: DMC Callant, AMCF Castelijns, JG De Vries.
- ↑ S. Coseri: phthalimides-N-oxyl (PINO) radical, a Powerful Catalytic Agent: Its Generation and Versatility Towards Various Organic substrate . In: Catal. Rev. Sci. Closely. tape 51 , no. 2 , 2009, p. 218-292 , doi : 10.1080 / 01614940902743841 .
- ^ Y. Ishii, K. Nakayama, M. Takeno, S. Sakaguchi, T. Iwahama, Y. Nishiyama: Novel Catalysis by N-Hydroxyphthalimide in the Oxidation of Organic Substrates by Molecular Oxygen . In: J. Org. Chem. Band 60 , no. 13 , 1995, pp. 3934-3935 , doi : 10.1021 / jo00118a002 .
- ↑ a b Discovery of a carbon radical producing catalyst and its application to organic synthesis. In: TCIMAIL, number 116. Tokyo Chemical Industry Co. Ltd., April 2003, accessed August 11, 2016 .
- ↑ BB Wentzel, MPJ Donners, PL Alsters, MC Feiters, RJM Nolte: N-Hydroxyphthalimide / cobalt (II) catalyzed low temperature benzylic oxidation using molecular oxygen . In: Tetrahedron . tape 56 , 2000, pp. 7797-7803 , doi : 10.1016 / S0040-4020 (00) 00679-7 .
- ↑ F. Minisci, C. Punta, F. Recupero, F. Fontana, GF Pedulli: Aerobic Oxidation of N-Alkylamides Catalyzed by N-Hydroxyphthalimide under Mild Conditions. Polar and Enthalpic Effects . In: J. Org. Chem. Band 67 , no. 8 , 2002, p. 2671-2676 , doi : 10.1021 / jo16398e .
- ↑ O. Fukuda, S. Sakaguchi, Y. Ishii: A new strategy for catalytic Baeyer-Villiger oxidation of KA-oil with molecular oxygen using N-hydroxyphthalimide . In: Tetrahedron Lett. tape 42 , no. 20 , 2001, p. 3479-3481 , doi : 10.1016 / S0040-4039 (01) 00469-5 .
- ↑ S. Sakaguchi, Y. Nishiwaki, T. Kitamura, Y. Ishii: Efficient catalytic alkane nitration with NO2 under air assisted by N-hydroxyphthalmide . In: Angew. Chem., Int. Edit. tape 40 , no. 1 , 2001, p. 222-224 , doi : 10.1002 / 1521-3773 (20010105) 40: 1 <222 :: AID-ANIE222> 3.0.CO; 2-W .
- ↑ Patent EP0664479A1 : Method of processing silver halide photographic lightsensitive material. Applied on December 6, 1994 , published on July 26, 1995 , applicant: Konica Corp., inventor: W. Ishikawa, T. Sampei.
- ↑ Patent US5332637 : Electrostatographic dry toner and developer compositions with hydroxyphthalimide. Applied August 31, 1993 , published July 26, 1994 , Applicant: Eastman Kodak Co., Inventor: JC Wilson, SM Bonser, HW Osterhoudt.