Ethosuximide
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| Structural formula without stereochemistry | |||||||||||||||||||
| General | |||||||||||||||||||
| Non-proprietary name | Ethosuximide | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 7 H 11 NO 2 | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| ATC code | |||||||||||||||||||
| Drug class | |||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 141.17 g · mol -1 | ||||||||||||||||||
| solubility |
soluble in ethanol (100 mg / ml) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Ethosuximide is an antispasmodic medicine used to treat epilepsy . Its chemical structure is similar to that of phenytoin , which is also used for anti-epileptic therapy .
Mechanism of action and area of application
Ethosuximide reduces voltage-dependent calcium currents by inhibiting the T-type calcium channels in the intralaminar nucleus of the thalamus .
It is mainly used for the treatment of generalized absences, especially of childhood (childhood absence epilepsy ). This form of epilepsy shows typical seizure patterns in the form of 3 Hz spike- wave complexes on the electroencephalogram . Ethosuximide is a first choice and has a super additive effect to valproic acid when combined with it. Ethosuximide is also indicated for myoclonus in adolescents ( impulsive petit mal ) when other drugs were not effective or were not tolerated.
The elimination takes place largely via the liver, the half-life ( half-life ) is around 48 to 60 hours, in children around 30 hours. Concomitant use of valproic acid can increase the half-life of ethosuximide by competitive inhibition of metabolism, whereas concomitant use of carbamazepine induces a higher plasma clearance .
Side effects
Depending on the dose, nausea, vomiting, hiccups, tiredness, headache, depression, movement disorders and psychoses can occur. Irrespective of the dose, various skin rashes including Stevens-Johnson syndrome and changes in the blood count have been described.
chemistry
Ethosuximide is a chiral drug with a stereocenter . The racemate , the 1: 1 mixture of the ( S ) and the ( R ) isomers, is used therapeutically .
| Enantiomers of ethosuximide | |
|---|---|
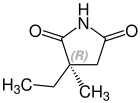 CAS no. 39122-20-8 |
 CAS no. 39122-19-5 |
Trade names
Petinimid (A, CH), Petnidan (D), Suxilep (D), Suxinutin (A)
Individual evidence
- ↑ a b c Datasheet Ethosuximide from Sigma-Aldrich , accessed on March 31, 2011 ( PDF ).
- ↑ Data sheet ETHOSUXIMIDE CRS (PDF) at EDQM , accessed on July 21, 2008.
- ↑ Ethosuximide seems to be the best choice , DAZ 23/2010.
- ↑ Ernst Mutschler , Gerd Geisslinger, Heyo K. Kroemer , Sabine Menzel, Peter Ruth: Mutschler drug effects. Pharmacology - Clinical Pharmacology - Toxicology. 10th edition. Wissenschaftliche Verlagsgesellschaft mbH, Stuttgart 2012, ISBN 3-80-472898-7 . P. 290.
- ↑ Rote Liste Service GmbH (Ed.): Rote Liste 2017 - drug directory for Germany (including EU approvals and certain medical devices) . Rote Liste Service GmbH, Frankfurt / Main, 2017, edition 57, ISBN 978-3-946057-10-9 , p. 182.