Fusaric acid
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
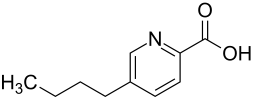
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Fusaric acid | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 10 H 13 NO 2 | ||||||||||||||||||
| Brief description |
colorless photosensitive crystals |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 179.21 g · mol -1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
108-109 ° C |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Fusaric acid is a picolinic acid - derivative , as the secondary metabolite of the plant-damaging fungi of the genus Fusarium and the type Gibberella fujikuroi is formed.
Fusaric acid has herbicidal , insecticidal and antibacterial effects. It is a wilt toxin for plants (causes premature wilting), but it is only slightly toxic to the animal organism. It inhibits dopamine β-hydroxylase (an enzyme that converts dopamine to noradrenaline ), has antihypertensive activity and influences the membrane properties of cells. It works partially synergistically with other mycotoxins such. B. Deoxynivalenol (DON). Therefore, their role as a mycotoxin is not yet fully understood.
history
Fusaric acid was first identified in 1934 by Teijiro Yabuta at the University of Tokyo as a metabolite of the rice-damaging fungus Gibberella fujikuroi ( Fusarium moniliforme ).
Individual evidence
- ↑ a b entry on fusaric acid. In: Römpp Online . Georg Thieme Verlag, accessed on December 29, 2014.
- ↑ a b Fusaric acid data sheet from Sigma-Aldrich , accessed on October 16, 2016 ( PDF ).
- ↑ Entry on fusaric acid in the ChemIDplus database of the United States National Library of Medicine (NLM), accessed on April 7, 2020.
- ↑ T. Yabuta, K. Kambe, T. Hayashi (1937): Biochemistry of the bakanae fungus. I. Fusarinic acid, a new product of the bakanae fungus. In: J. Agric. Chem. Soc. Jpn. Vol. 10, pp. 1059-1068.
