Antimonic acid
| Structural formula | ||||||||
|---|---|---|---|---|---|---|---|---|
|
||||||||
| General | ||||||||
| Surname | Antimonic acid | |||||||
| other names |
Hexahydroxidoantimony (V) acid |
|||||||
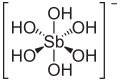
| Molecular formula | H [Sb (OH) 6 ] | |||||||
| External identifiers / databases | ||||||||
|
||||||||
| properties | ||||||||
| Molar mass | 224.80 g mol −1 | |||||||
| pK s value |
2.55 |
|||||||
| safety instructions | ||||||||
|
||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||
Of the hypothetical ortho-antimonic acid H 3 SbO 4 , only one hexahydroxidoantimonic acid stabilized by the additional addition of two water molecules is known in dilute aqueous solution with the empirical formula H [Sb (OH) 6 ]. The weak monobasic acid forms isolable and stable salts , the hexahydroxidoantimonates (V). The best known is the water-soluble potassium hexahydroxidoantimonate (V) for the detection of sodium by the formation of the insoluble sodium hexahydroxidoantimonate. The ortho-, meta- and pyro-antimonic acids, which are poor in water, cannot be produced, since insoluble antimony pentoxide is deposited when the water-rich antimonic acid solution is dehydrated . Salts of the less watery antimonic acids can be isolated by dry processes.
Antimonic acid is formed by dissolving the sparingly soluble antimony pentoxide Sb 2 O 5 in water. It can be reduced to antimony trioxide (Sb 2 O 3 ) by using stronger reducing agents .
Individual evidence
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 .
- ^ Entry on antimony compounds in the GESTIS substance database of the IFA , accessed on February 1, 2016 (JavaScript required)
- ↑ Not explicitly listed in Regulation (EC) No. 1272/2008 (CLP) , but with the specified labeling it falls under the group entry antimony compounds, with the exception of the tetroxide (Sb2O4), pentoxide (Sb2O5), trisulphide (Sb2S3), pentasulphide (Sb2S5) and those specified elsewhere in this Annex in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .


