Lactates
As Lactate (including lactate ) refers to salts and esters of lactic acid .
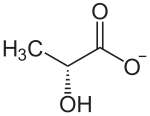
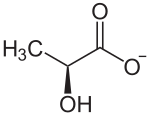
Since lactic acid occurs in two enantiomeric forms, there are also two corresponding forms of its anion, which are usually referred to as D and L form according to their orientation in the Fischer projection (The Fischer projection is shown vertically, however, with the more highly oxidized Group above). Lactate formed in the human body is only available in the clockwise L (+) form.
Salts
| Metabolic intermediate | |
|---|---|
|
|
| Structural formulas of the D- lactate ion (left) and the L- lactate ion (right) | |
| General | |
| Surname |
Lactate |
| properties | |
| Molecular formula | C 3 H 5 O 3 - |
| Molar mass | 89.07 g · mol -1 |
| Ion at pH 7 |
Mono anion |
| Identifiers | |
| CAS number |
113-21-3 |
| PubChem | |
Lactic acid is a strong carboxylic acid that dissociates strongly under physiological conditions . The anion has the constitutional formula CH 3 –CHOH – COO - and is known as lactate .
Lactate in the human organism
The most common lactate found in the human body is sodium lactate. It is mainly formed in the skeletal muscles.
The breakdown of glucose or glycogen to pyruvate in glycolysis is coenzyme NAD + to NADH / H + reduced. In order for glycolysis to take place, it must be in an oxidized form, as NAD + . Only in this way can it function as an electron acceptor in the oxidation of glyceraldehyde-3-phosphate to 1,3-bisphosphoglycerate by glyceraldehyde-3-phosphate dehydrogenase . Since not all of the NADH / H + that accumulates in muscle fibers with poor mitochondria can be oxidized so quickly with increasing stress , the organism helps itself by reducing pyruvate to lactate. Here, NADH / H + to NAD + is reoxidized. The breakdown of glucose to lactate is also known as homofermentative lactic acid fermentation .
In the skeletal muscle, skin, intestinal mucosa, blood cells, kidneys and brain, about 0.7-1.3 mmol of lactate are formed together per hour.
At rest or during light work, around 75% of the energy required is obtained from fats and only 25% from carbohydrates. At 38.9 kJ g −1, fats have a higher calorific value than carbohydrates (17.2 kJ g −1 ). With increasing stress, carbohydrates are increasingly used, as they provide significantly more adenosine triphosphate (ATP) per unit of time ( FFS 0.4 mmol min −1 , glycogen 1.0 mmol min −1 , glucose 3.0 mmol min −1 ).
The lactate is already broken down during exercise. To do this, its oxidation to pyruvate is fundamentally necessary. Depending on the load, increasing amounts of it are supplied to the oxidative energy supply, the literature speaks of up to 83%. The lactate is used in both the skeletal muscles and the heart muscle. The heart covers up to 60% of its energy requirements under stress.
In contrast, lactate is built up in the liver with the use of energy via pyruvate to glucose. The resynthesis of lactate to glucose is called gluconeogenesis .
With higher and highest loads, fats take a back seat as a source of energy. There are degraded in the active muscles increasingly glucose and glycogen to pyruvate to lactate is reduced due to the lack of NAD (and thus the NADH / H + to NAD + oxidized). Since the system of lactate utilization is overwhelmed, the lactate accumulates in the muscles, the reaction equilibrium shifts and glycolysis is strongly inhibited or comes to a standstill. This situation can be measured by a kink in the lactate performance curve (individual anaerobic threshold ) that can be determined, for example, using differential calculation .
The level of lactate in the blood is the result of the constant production and elimination of lactate throughout the body. With maximum exposure in the short-term range (e.g. 1000 m time trial and keirin in cycling, swimming, 400 m run) lactate levels of up to 35 mmol l −1 can arise.
The lactate is in the Lactatleistungsdiagnostik used. The so-called individual anaerobic threshold can be determined here by determining the course of the lactate value under different loads. This plays an important role, for example, in performance diagnostics based on lactate level tests for determining individual performance.
Lactate in clinical medicine
In medicine, lactate is used as an ischemia marker because it is formed in the tissue when there is a lack of oxygen. One possible cause is, for example, intestinal ischemia due to the blockage of blood vessels leading to the intestine. In a phosphorus poisoning in atrophy of the liver (liver fading), osteomalacia and trichinosis lactate is detectable in the urine.
- Normal values in the blood: 5–20 mg / dl (corresponds to 0.55–2.2 mmol / l);
- CSF normal range: 11–19 mg / dl;
- Joint puncture normal range: 9–16 mg / dl
Lactate is mainly determined in intensive care medicine:
- for evaluating the course of circulatory shock and poisoning
- for the detection of tissue hypoxia
- to clarify unclear metabolic acidoses
Otherwise:
- with joint effusions
- in sports medicine for assessing physical performance and training control by determining the anaerobic threshold (approx. 4 mmol / l).
Lactate in microorganisms
Microorganisms also produce lactate during lactic acid fermentation. In contrast to humans, these can also form the D -isomer through a D - lactate dehydrogenase .
Ester
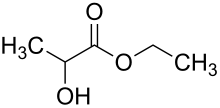
Lactates (CH 3 -CHOH-COOR) are also called lactates. Ethyl lactate (lactic acid ethyl ester) is the most important representative of these esters, which is used, among other things, as a solvent. Another representative is butyl lactate (lactic acid butyl ester ).
presentation
The lactates are accessible through esterification of lactic acid with alcohols (R-OH).
- Lactic acid reacts in a condensation reaction with an alcohol to form a lactic acid ester and water.

Due to the alcohol function also contained in the lactic acid molecule, intermolecular esterification to a lactide can also take place. Lactides, in turn, are the starting substances for polylactides ( plastics ).
Use as a food additive
Lactic acid esters of mono- and diglycerides of fatty acids are used as food additives and are marked with the E number E 472b.
literature
- Georg Löffler, Petro E. Petrides, Peter C. Heinrich (Eds.): Biochemistry & Pathobiochemistry. 8th, completely revised edition. Springer Medicine, Heidelberg 2007, ISBN 978-3-540-32680-9 , p. 358 f.
- Horst de Marées : Exercise Physiology. 9th, completely revised and enlarged edition, corrected reprint. Sportverlag Strauss, Cologne 2003, ISBN 3-89001-010-5 .
- Georg Neumann, Arndt Pfützner, Anneliese Berbalk: Optimized endurance training. Meyer & Meyer, Aachen 1998, ISBN 3-89124-498-3 .
Individual evidence
- ↑ Neumann, Pfützner, Berbalk: Optimized endurance training. 1998, p. 83.
- ↑ a b Patrick Wahl, Wilhelm Bloch, Joachim Mester: Modern ways of looking at lactate: Lactate is an overestimated and at the same time underestimated molecule. In: Swiss journal for sports medicine and sports traumatology. Vol. 57, No. 3, 2009, pp. 100–107, here p. 104, online full-text access (accessed December 9, 2015; PDF; 206 kB).
- ↑ Description of lactic acid esters of mono- and diglycerides as a food additive
Web links
- VB Gerritsen: Another dark horse
