Butyl lactate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
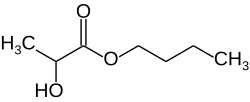
|
||||||||||||||||
| Simplified structural formula without stereochemistry | ||||||||||||||||
| General | ||||||||||||||||
| Surname | Butyl lactate | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 7 H 14 O 3 | |||||||||||||||
| Brief description |
colorless liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 146.19 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.97 g cm −3 |
|||||||||||||||
| Melting point |
-43 ° C |
|||||||||||||||
| boiling point |
170 ° C |
|||||||||||||||
| Vapor pressure |
0.53 mbar (20 ° C) |
|||||||||||||||
| solubility |
|
|||||||||||||||
| Refractive index |
1.421 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| MAK |
Switzerland: 5 ml m −3 or 30 mg m −3 |
|||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Lactic acid butyl ester , also butyl lactate , is the butyl ester of lactic acid .
Extraction and presentation
Butyl lactate can be obtained, for example, by reacting calcium or sodium lactate with 1-butanol in benzene in the presence of sulfuric acid. This creates a racemate from the D and L forms . The pure D or L form in each case can be prepared by reacting zinc ammonium D or zinc ammonium L lactate with 1-butanol in the presence of hydrochloric acid .
properties
Butyl lactate is a slightly volatile, colorless liquid that is soluble in water.
use
Butyl lactate is used in the manufacture of nanoparticles and as a solvent (e.g. for nitrocellulose ).
Butyl lactate has also been used as a fragrance since the 1930s.
safety instructions
The vapors of butyl lactate can form an explosive mixture with air ( flash point 61 ° C, ignition temperature 380 ° C).
Individual evidence
- ↑ a b c d e f g h i j k l m Entry on butyl lactate in the GESTIS substance database of the IFA , accessed on January 9, 2019(JavaScript required) .
- ↑ a b Entry on butyl lactate in the Hazardous Substances Data Bank , accessed on March 9, 2015.
- ↑ a b data sheet Butyl lactate, 98% from Sigma-Aldrich , accessed on March 9, 2015 ( PDF ).
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 138-22-7 or butyl lactate ), accessed on November 2, 2015.
- ↑ George A. Burdock: Fenaroli's Handbook of Flavor Ingredients, Fifth Edition: . CRC Press, 2004, ISBN 978-1-4200-3787-6 , pp. 211 ( limited preview in Google Book search).
- ↑ Rakesh Kumar, Sanjay M. Mahajani: Esterification of Lactic Acid with n-Butanol by Reactive Distillation. In: Industrial & Engineering Chemistry Research. 46, 2007, p. 6873, doi : 10.1021 / ie061274j .
- ↑ unknown: Butyl lactate . Monographs on Fragrance Raw Materials. In: Food and Cosmetics Toxicology . tape December 17 , 1979, pp. 727 , doi : 10.1016 / s0015-6264 (79) 80014-0 (English, PDF ).
