Calcium lactate
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
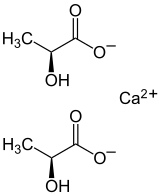
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Calcium lactate | |||||||||||||||||||||
| other names | ||||||||||||||||||||||
| Molecular formula | C 6 H 10 CaO 6 | |||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 218.22 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Calcium lactate (also calcium lactate) is the calcium - salt of lactic acid .
Occurrence
Calcium lactate occurs naturally in old cheese, where it was formed by bacteria during ripening from the lactic acid originally contained and partly crystallizes on the surface. The double salt of calcium lactate and calcium gluconate is called calcium lactate gluconate .
Extraction and presentation
Calcium lactate is synthesized from lactic acid in industrial production.
use
In the food industry it is added to foods as a humectant and acid regulator . Calcium lactate reacts with pectin , which is contained in fruit peels , to form insoluble calcium pectinate , which hardens fruits and vegetables. It is therefore used to treat fruit that is sold cut in order to extend its shelf life and ensure that the pulp remains firm for a longer period of time. It is approved in the EU as a food additive with the number E 327 for all foods that may contain additives. E 327 is not permitted for food from " organic agriculture " .
Calcium lactate is generally considered to be harmless, only for allergy sufferers is a concern. It is used as a mineral supplement in dietary foods and is also used medicinally to combat calcium deficiency . Another medicinal use is as an antacid .
It is also used in mouthwashes and dental care chewing gum. In conjunction with xylitol , it can promote the remineralization of tooth enamel.
Web links
Individual evidence
- ↑ Entry on CALCIUM LACTATE in the CosIng database of the EU Commission, accessed on May 22, 2020.
- ↑ a b Data sheet L - (+) - Lactic Acid, Calcium Salt (PDF) from Calbiochem, accessed on December 8, 2015.
- ↑ EG-ÖKO-VO 834/2008 Annex 8a.
- ↑ R. Sudaa, T. Suzukia, R. Takiguchib, K. Egawab, T. Sanob, K. Hasegawa: The Effect of Adding Calcium Lactate to Xylitol Chewing Gum on remineralization of Enamel Lesions . In: Caries Research . 40, No. 1, 2006, pp. 43-46. doi : 10.1159 / 000088905 . PMID 16352880 .