Sodium lactate
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
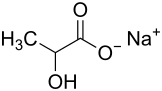
|
||||||||||||||||||||||
| Mixture of stereoisomers - structural formula without stereochemistry | ||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Sodium lactate | |||||||||||||||||||||
| other names | ||||||||||||||||||||||
| Molecular formula | NaC 3 H 5 O 3 | |||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 112.06 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| density |
1.33 g / ml, 1.31 g / ml (60 percent syrup) |
|||||||||||||||||||||
| Melting point |
161-162 ° C |
|||||||||||||||||||||
| solubility |
miscible with water |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Sodium lactate (sodium lacticum) is the sodium salt of lactic acid with the formula NaC 3 H 5 O 3 .
properties
Sodium lactate is a colorless solid with a slightly salty taste.
Extraction
Sodium lactate is produced (like lactates in general) by fermenting a source of sugar such as corn or beet and then neutralizing the lactic acid produced.
Commercially used lactic acid is mostly fermented from dairy-free raw materials such as corn starch , potatoes or molasses . Furthermore, sugar or tapioca are used. The starter culture for fermentation can contain dairy products. Lactic acid is partly fermented from milk products such as whey and lactose , which contain 4.8% lactose at 6.5% solids. In turn, lactic acid from dairy products is often used in dairy products such as ice cream and double cream cheese . Typically, whey is used to make lactic acid when the whey itself is a waste from the production of certain dairy products.
use
Food technology
Sodium lactate is used in food technology as an acidity regulator , humectant , melting salt or as a firming agent. For use in food, it is offered as a naturally liquid product or in powder form. In the EU, it is approved as a food additive with the number E 325 for all foodstuffs permitted for additives - including " organic " products - without a maximum quantity restriction ( quantum satis ). Sodium lactate is harmless in the case of milk intolerance or allergy, as milk is usually not used as a raw material and otherwise does not contain any milk protein.
Cosmetics, paper and textile production
Sodium lactate ( SODIUM LACTATE , INCI ) can be used in shampoos and other similar items such as liquid soap as it is an effective humectant.
It is still used in cosmetics, as well as in the manufacture of paper and textiles and in tobacco processing.
medicine
Sodium lactate is used in combination with hypertensive sympathomimetics to treat arrhythmias caused by overdosing on Class I antiarrhythmics .
A 50 percent solution (according to the pharmacopoeia) is used as the medicinal substance, which has a pH value of 6.5 to 9.0 and is colorless, odorless and slightly viscous.
history
As early as 1836 it was recognized that sodium lactate was a salt of a weak acid rather than a base, and it was already known that the lactate must be metabolized in the liver before the sodium can have a measurable effect on the pH value.
In the world wars it was used as a substitute for glycerine in order to save it for war purposes.
Individual evidence
- ↑ Entry on E 325: Sodium lactate in the European database for food additives, accessed on June 27, 2020.
- ↑ Entry on SODIUM LACTATE in the CosIng database of the EU Commission, accessed on May 26, 2020.
- ↑ a b c Entry on Sodium lactate at ChemBlink , accessed on April 18, 2012.
- ↑ Sodium Lactate, Powder ( Memento from August 24, 2012 in the Internet Archive ) (PDF; 181 kB), lotioncrafter.com
- ↑ Sodium DL-lactate ≥99% data sheet from Sigma-Aldrich , accessed on February 15, 2012 ( PDF ).
- ^ A b c Barrie Silberberg: The Autism and ADHD Diet: A Step-by-Step Guide to Hope and Healing by Living Gluten Free and Casein Free (GFCF) and Other Interventions . Sourcebooks, Inc., 2009, ISBN 1402218451 , p. 119.
- ↑ a b c Alisa Marie Fleming: Go Dairy Free: The Guide and Cookbook for Milk Allergies, Lactose Intolerance, and Casein-free Living . Go Dairy Free, 2008, ISBN 0979128625 , p. 90.
- ^ MD Ranken, RC Kill: Food industries manual . Springer, 1997, ISBN 0751404047 , p. 125.
- ^ Inamdar: Biochemical Engineering: Principles And Concepts . PHI Learning Pvt. Ltd., 2009, ISBN 8120336771 , p. 254.
- ↑ Restricted additives , Annex 4 (to § 5 Paragraph 1 and § 7) of the Additive Admissions Ordinance
- ↑ Alice Willitts, Deborah Carter: Food allergy & your child . Class Publishing Ltd, 2007, ISBN 1859591868 , p. 85: "The following ingredients do not contain milk protein and need not be avoided by people allergic to milk:… Sodium lactate"
- ^ Anthony Trevor, Bertram Katzung, Susan Masters: Katzung & Trevor's Pharmacology Examination and Board Review , 8e. Edition, Go Dairy Free, 2008, ISBN 0071488693 , p. 126.
- ^ Lewis C. Mills, John Henry Moyer, Hahnemann Medical College and Hospital of Philadelphia: Shock and hypotension: pathogenesis and treatment: the twelfth Hahnemann symposium . Grune & Stratton, 1836, p. 369.
- ^ Albert A. Dietz, Ed. F. Degering, HH Schopmeyer: Physical Properties of Sodium, Potassium, and Ammonium Lactate Solutions . In: Industrial & Engineering Chemistry . tape 33 , no. 11 , 1941, pp. 1444-1447 , doi : 10.1021 / ie50383a027 (English).