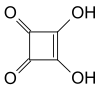
Squaric acid
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Squaric acid | |||||||||||||||
| other names |
3,4-dihydroxycyclobut-3-ene-1,2-dione |
|||||||||||||||
| Molecular formula | C 4 H 2 O 4 | |||||||||||||||
| Brief description |
beige solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 114.06 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.82 g cm −3 |
|||||||||||||||
| Melting point |
Decomposes at 293 ° C |
|||||||||||||||
| Vapor pressure |
<0.01 hPa at 20 ° C |
|||||||||||||||
| pK s value |
|
|||||||||||||||
| solubility |
readily soluble in water (20 g l −1 at 20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
The squaric acid is a derivative of cyclobutene , belongs to the group of oxocarbons and is related to the MONILIFORMIN ( moniliformin ), the most acidic known natural substance . The salts of squaric acid are called quadratates.
presentation
Squaric acid was first prepared by reacting chlorotrifluoroethylene with zinc . The resulting perfluorocyclobutene is converted to 1,2-diethoxy-3,3,4,4-tetrafluoro-1-cyclobutene in ethanol . Acid hydrolysis finally produces squaric acid.
properties
The name square acid is actually incorrect because the four carbon atoms do not form a square due to different C – C bond lengths . In the crystalline state, the planar C 4 O 4 subunits arrange themselves via hydrogen bonds to form a flat layer.
The high acidity (pK s 1 = 1.5, pK s 2 = 3.4) of the squaric acid can, similarly to z. B. in delta , croconic or rhodizonic acid , explain by resonance stabilization of the anion . The dianion of squaric acid is completely symmetrical and shows no different C – C or C – O bond lengths:
As with the anions of the other acids mentioned above ( oxocarbons ), it is an aromatic system with 2 π electrons.
use
Squaric acid is used in chemistry, industry and medicine.
chemistry
- Squaric acid was used as a building block for the synthesis of new poly-alkoxo-oxometallate derivatives , such as. B. the K 4 [V IV 12 O 12 (OCH 3 ) 16 (C 4 O 4 ) 6 ] is used. For this purpose, methyl ortho- vanadate was reacted with squaric acid and potassium hydroxide under solvothermal conditions.
- Squaric acid was also used to prepare new organotin compounds. In 2004, for example, by reacting trimethyltin chloride , tributyltin chloride or dimethyltin dichloride with disodium squared, the synthesis of bis (trimethyl), bis (tributyltin) and dimethyltin squares was achieved.
Industry
Dyes derived from squaric acid, the squaraine, are of increasing industrial importance .
medicine
Squaric acid can be used to treat warts. In the treatment known as immunotherapy, an irritant concentration (2-3% solution in acetone ) is applied first. If an immunological reaction occurs, the wart can be treated with a much lower concentrated solution.
In addition to diphenylcyclopropenone (DPCP), dibutyl squared ester (SADBE) is used to treat hair loss . As part of topical immunotherapy for therapy-resistant alopecia areata or alopecia totalis, targeted contact sensitization of the scalp with diluted squaric acid solution takes place.
The use of squaric acid as a linker between target structures and chelators for use in molecular imaging is the subject of current research.
See also
Individual evidence
- ↑ a b c d e f g Data sheet squaric acid (PDF) from Merck , accessed on December 26, 2019.
- ^ A b Robert West, David L. Powell: “New Aromatic Anions. III. Molecular Orbital Calculations on Oxygenated Anions “, in: J. Am. Chem. Soc. , 1963 , 85 (17), pp. 2577-2579; doi : 10.1021 / ja00900a010 .
- ↑ Sidney Cohen, John R. Lacher, Joseph D. Park: "Diketocyclobutenediol", in: J. Am. Chem. Soc. , 1959 , 81 (13), pp. 3480-3480; doi : 10.1021 / ja01522a083 .
- ↑ Entry on squaric acid. In: Römpp Online . Georg Thieme Verlag, accessed on June 15, 2014.
- ↑ Johann Spandl, Irene Brüdgam, Hans Hartl: "From small building blocks to new poly-alkoxo-oxometallate derivatives: synthesis and structure elucidation of K 4 [V IV 12 O 12 (OCH 3 ) 16 (C 4 O 4 ) 6 ], Cs 10 [V IV 24 O 24 (OCH 3 ) 32 (C 4 O 4 ) 12 ] [V IV 8 O 8 (OCH 3 ) 16 (C 2 O 4 )] and M 2 [V IV 8 O 8 (OCH 3 ) 16 (V IV OF 4 )] (M = [N ( n Bu) 4 ] or [NEt 4 ]) “, in: Z. anorg. allg. Chem. , 2003 , 629 (3), pp. 539-544; doi : 10.1002 / zaac.200390087 .
- ↑ Asrial: Synthesis and Characterization of New Organotin Compounds , Cuvillier Verlag, Göttingen 2004, ISBN 3-86537-259-7 ( limited preview in Google book search).
- ↑ Nooshin Bagherani: Efficacy of squaric acid dibutyl ester in treatment of recalcitrant warts in children. In: Dermatologic Therapy . Volume 28, No. 5, 2015, p. 330, doi: 10.1111 / dth.12234 .
- ↑ P. Freyschmidt-Paul, R. Happle, R. Hoffmann: Alopecia areata - Clinic, pathogenesis and rational therapy of a T-cell-mediated autoimmune disease. In: The dermatologist . Vol. 54, 2003, pp. 713-722; doi : 10.1007 / s00105-003-0560-z .
- ↑ Sebastian Görres: Development of new ligands for fibrosis imaging using PET and SPECT . In: unpublished dissertation . Hanover 2016, p. 46 ( d-nb.info ).
- ^ German Society for Nuclear Medicine eV: German Society for Nuclear Medicine eV Accessed on September 21, 2018 .


