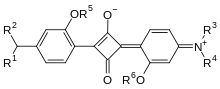
Squaraine
As squaraines refers to a class of intensive dyes whose base since 1980 sq acid and their esters are. In German-speaking countries they are therefore sometimes referred to as squaric acid dyes , older names - which are no longer used today - are tetracyclotrimethine , cyclobutenediylium dyes or substituted oxocyclobutenolates.
General
Because of their unusual structural, electronic and photophysical properties, squaraine dyes are not only of scientific interest. They show a high thermal and photochemical stability and were therefore used shortly after their discovery as sensitizers for inorganic photoconductors . Some time later, squaraines were also used as chemical sensors and as xerographic photoreceptors . Today there is a particular interest in incorporating squaraine structures into conjugated polymers . This should pave the way for new conductive or photoconductive materials.
Definition and discovery
The squaraine were discovered in the mid- 1960s by two research groups simultaneously and independently of one another. Your name Squarain is a combination of Squar ic acid , the English term for squaric acid , and Bet ain , which means zwitterionic molecules with a negative and a positive charge center.
The squaraines are always 1,3-dicondensation products of squaric acid with nucleophilic compounds and thus all contain a cyclobutenediylium-1,3-diolate unit. As nucleophiles, for. B. primary and secondary amines, electron-rich aromatic and heteroaromatic compounds are used.
This basic structure of the squaraine also explains their intense colors. From a formal point of view, the central four- ringed squaraine corresponds to a Hückel aromatic with two π electrons. Electron conjugation across the entire, planar molecule is thus possible. The two usually electron-rich aryl radicals push electrons into the electron-poor four-membered ring. The result is a donor-acceptor-donor system with strong intramolecular charge-transfer effects .
Depending on the structure, the connections are divided into symmetrical and asymmetrical squaraine. As asymmetrical squaraines are called squaric acid derivatives which have different substituents in the 1- and 3-position, symmetrical squaraines, on the other hand, have the same substituents.
Synthesis of the squaraine
Various ways of synthesizing squaraine are discussed in the literature. The three most important are presented here.
Direct aromatic squaric acid condensation
A squaric acid half-ester is formed by heating squaric acid in an alcohol (mostly butanol ) . The aromatic nucleophile attacks the vinylogous ester carbon and substitutes the alkoxy group with elimination of the corresponding alcohol. A further nucleophile can then be added to this 1: 1 adduct in the trans position to the first nucleophile on the carbonyl carbon of the four-membered ring. The decisive disadvantage of this synthetic route is the limited variety of synthesis, which results from the need to use electron-rich, sterically less demanding aryls.
Ketene dimerization
The second way is based on a dimerization of ketenes . These are generated in situ by dehydrochlorination of phenylacetyl chlorides. The resulting ketenes react in a [2 + 2] cycloaddition to form a dihydrosquaraine, from which the desired squaraine is obtained by oxidation . The advantage of this synthetic route is that lower-electric aryl systems can also be used. So z. B. the synthesis of bis (4-methoxyphenyl) squaraine only in this way.
Synthesis via half-squaraine adducts
Half-squaraine (i.e. 4-aryl-1-hydroxy-cyclobutene-3,4-diones) can be represented in three ways; the desired squaraine is then obtained by condensation with activated aromatics.
- In a [2 + 2] cycloaddition, phenyl ketene is reacted with tetraethoxyethylene; the resulting intermediate is hydrolyzed .
- The second way is the Lewis acid catalyzed Friedel-Crafts reaction between squaric acid dichloride and an aromatic with subsequent hydrolysis.
- The third possibility is a nucleophilic 1,2-addition of lithium aryl compounds to a carbonyl group of the respective squaric acid ester, followed by oxy-elimination and subsequent hydrolysis.
Applications of the squaraine
The first squaraine were discovered in 1965 by Treibs and Jacob. They condensed squaric acid with substituted pyrroles and obtained deep-colored, thermally highly stable compounds as a result. Then squaraines followed on the basis of N , N dialkyl aniline derivatives. The bis ( N , N- dialkylaminophenyl) squaraines which result from this represent an important class to this day . Squaraines obtained by condensation with pyrazolone , benzothiophene and barbituric acid derivatives followed.
Squaraine was originally used as a sensitizer for ZnO photoconductors; in the 1970s, their possible applications in the field of solar technology were investigated. Since squaraines show intense absorption in the near infrared range, they can also be used in optical storage media or as laser dyes.
In the 1990s, research on squaraine syntheses was revived because of its attractive photophysical properties. By introducing more highly substituted aromatics with additional hypsochromic or bathochromic groups in the ortho- and / or meta-position, properties such as solubility , crystal formation or light absorption can be controlled. The aim is to improve the technical photoconductivity and other xerographic properties.
Today squaraine are mainly used as fluorescent dyes in DNA sequence analysis and as materials for nonlinear optics (NLO) . Since the late 1990s, research has concentrated on the synthesis of oligo- and polysquaraines as well as on chiral squaraines.
Individual evidence
- ↑ a b A. H. Schmidt. In: Synthesis 1980, pp. 961-994.
- ^ H. Kampfer, KE Verhille. US patent 3617270, 1971.
- ↑ a b K. Y. Law. In: J. Phys. Chem. 92: 4226-4231 (1988).
- ↑ YG Isgor, EU Akkaya. In: Tetrahedron Lett. 38 (1997), pp. 7417-7420.
- ↑ EU Akkaya, S. Turkyilmaz. In: Tetrahedron Lett. 38 (1997), pp. 4513-4516.
- ^ KY Law, FC Bailey: Squaraine chemistry. Synthesis of bis (4-dimethylaminophenyl) squaraine from dialkyl squarates. Mechanism and scope of the synthesis . In: Canadian Journal of Chemistry . 64, 1986, pp. 2267-2273, doi : 10.1139 / v86-372 .
- ^ DG Farnum, JR Johnson, RE Hess, TB Marshall, B. Webster. In: J. Am. Chem. Soc. 87 (1965), pp. 5191-5197.
- ↑ a b D. G. Farnum, B. Webster, AD Wolf. In: Tetrahedron Lett. 1968, pp. 5003-5006.
- ↑ a b M. AB Block: Synthesis and characterization of polysquarain structures. Diploma thesis, FU Berlin 2002.
- ↑ KY Law, Bailey FC. In: J. Org. Chem. 57 (1992), pp. 3278-3286.
- ^ A b K.Y. Law, FC Bailey: Squaraine chemistry. A new approach to symmetrical and unsymmetrical photoconductive squaraines. Characterization and solid state properties of these materials . In: Canadian Journal of Chemistry . 71 (4), 1993, pp. 494-505, doi : 10.1139 / v93-070 .
- ↑ a b D. Bellus. In: J. Am. Chem. Soc. 100 (1978), p. 8026.
- ↑ a b G. Maahs, P. Hegenberg. In: Angew. Chem. 78: 927-931 (1966).
- ^ A b M. W. Reed, DJ Pollart, ST Perri, LD Foland, HW Moore ,. In: J. Org. Chem. 53 (1988), pp. 2477-2482.
- ↑ A. Treibs, K. Jacob. In: Angew. Chem. 77 (1965), pp. 680-681.
- ^ HE Sprenger, W. Ziegenbein. In: Angew. Chem. 78, pp. 937-938 (1966).
- ^ HE Sprenger, W. Ziegenbein. In: Angew. Chem. 80 (1968), pp. 541-546.
- ^ KY Law. In: Chem. Rev. 93 (1993), pp. 449-486.
- ↑ Manuela Schiek, Oriol Arteaga, Arne Lützen, Frank Balzer, Stefanie Brück: Giant intrinsic circular dichroism of prolinol-derived squaraine thin films . In: Nature Communications . tape 9 , no. 1 , June 20, 2018, ISSN 2041-1723 , p. 2413 , doi : 10.1038 / s41467-018-04811-7 ( nature.com [accessed April 19, 2019]).