Seignette salt
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
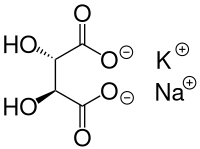
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Seignette salt | ||||||||||||||||||
| other names | |||||||||||||||||||
| Molecular formula | C 4 H 4 KNaO 6 (anhydrous) | ||||||||||||||||||
| Brief description |
colorless solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | |||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.79 g cm −3 |
||||||||||||||||||
| Melting point |
70–80 ° C (tetrahydrate) |
||||||||||||||||||
| boiling point |
Decomposition at 220 ° C |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Seignette salt is a salt of tartaric acid . The French pharmacist after whom it was named, Elie Seignette (1632–1698), discovered it between 1648 and 1660. It is a double salt containing potassium and sodium ions, now correctly called potassium - sodium - tartrate- tetrahydrate ( KNaC 4 H 4 O 6 · 4 H 2 O). The tartrates are the salts of tartaric acid, the Seignette salt is derived from the two bases potassium and sodium hydroxide .
The Seignette salt was discovered in La Rochelle and is therefore also called Rochelle Salt. Outdated names are tartrate of soda potassium or soda tartar. The Latin names that are important because of the use in pharmacy (mild emetic ) are Tartarus natronatus , Natrokali tartaricum , Kalium tartaricum natronatum (French: Tartrate de soude et de potasse, Sel de seignette, English Tartarated soda, Salt of seignette).
Ferroelectricity was discovered at the Seignette salt .
Manufacturing
Potassium sodium tartrate can be made from tartar and soda by adding tartar to a solution of soda until no more carbon dioxide escapes . After the filtered lye has been evaporated in the steam bath, beautiful crystals are formed which are dried at very low heat, as otherwise they lose their transparency or transparency due to the loss of crystal water .
Properties and use
The crystals form large, water-white, rhombic columns. The salt melts in its crystal water when heated, but is otherwise quite air-resistant. It dissolves in very little water, has a mild, salty taste and is used as a cooling, mild laxative, e.g. B. applied as Seidlitz powder. In analytical chemistry it is used as an aid for the detection of reducing sugars with Fehling's solution II or with Nylander's reagent . This makes use of the fact that it is a good chelator ( complexing agent ) and complexes copper or bismuth ions and binds them in the process.
When used as a chelator , instead of the salt of Seignette, another tartrate can be used, which forms an uncharged molecule with the corresponding atoms, as long as this is not itself a suitable ligand for the complex bond with the Seignette salt. The fact that the commercially available Seignette salt is composed of potassium sodium tartrate is related to the cost of this product. Potassium hydrogen tartrate occurs very frequently in nature and can be obtained in a simple manner. The carbonate of an alkali metal required for metering and neutralization is also relatively cheap as soon as sodium carbonate is used.
It was used to make the crystal pickup because of its piezoelectric property .
It is approved in the EU as a food additive with the number E 337 (mostly used as an acidity regulator ).
Web links
Individual evidence
- ↑ Entry on E 337: Sodium potassium tartrate in the European database on food additives, accessed on June 27, 2020.
- ↑ Entry on POTASSIUM SODIUM TARTRATE in the CosIng database of the EU Commission, accessed on May 4, 2020.
- ↑ a b c d Entry on potassium sodium tartrate in the GESTIS substance database of the IFA , accessed on February 3, 2018(JavaScript required) .
- ↑ a b c Entry on potassium sodium (R, R) tartrate. In: Römpp Online . Georg Thieme Verlag, accessed on May 12, 2014.
- ↑ It is often wrongly ascribed to his son Pierre Seignette . Article “Elie Seignette”, In: Pötsch u. a. Lexicon of important chemists , Harri Deutsch 1989.