Lauric acid
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
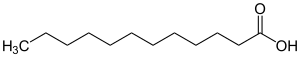
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Lauric acid | |||||||||||||||||||||
| other names |
Dodecanoic acid ( IUPAC ) |
|||||||||||||||||||||
| Molecular formula | C 12 H 24 O 2 | |||||||||||||||||||||
| Brief description |
colorless needles |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 200.32 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| density |
0.88 g cm −3 |
|||||||||||||||||||||
| Melting point |
44 ° C |
|||||||||||||||||||||
| boiling point |
298 ° C |
|||||||||||||||||||||
| Vapor pressure |
2.3 · 10 −3 Pa (298 K) |
|||||||||||||||||||||
| solubility |
|
|||||||||||||||||||||
| Refractive index |
1.4183 (82 ° C) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||||||||
Lauric acid (systematic name: dodecanoic acid ) is a saturated fatty and carboxylic acid . It is derived from the alkane n - dodecane . The name comes from the bay laurel ( Laurus nobilis ), the fruits of which produce a fatty oil that mainly contains lauric acid. Their salts and esters are called laurates (systematically also dodecanoates ).
properties
Lauric acid is a white solid that melts at 44 ° C. Lauric acid is widely used as a fatty acid component of triglycerides , for example in coconut fat (41 to 46%) and palm kernel oil (41 to 45%).
use
Lauric acid is used technically in the manufacture of soaps , as an additive in rolling oils in the aluminum industry.
Related substances
Sodium lauryl sulfate (SLS or SDS) is the sodium salt of the lauric alcohol monoester of sulfuric acid. It is an anionic surfactant that is used as a detergent . Sodium dodecyl poly (oxyethylene) sulfate (sodium lauryl ether sulfate) is also a commonly used anionic surfactant.
Individual evidence
- ↑ a b c d Entry on dodecanoic acid. In: Römpp Online . Georg Thieme Verlag, accessed on September 30, 2014.
- ↑ a b c d e Entry on lauric acid in the GESTIS substance database of the IFA , accessed on January 10, 2017(JavaScript required) .
- ↑ CD Cappa, ER Lovejoy, AR Ravishankara: Evaporation Rates and Vapor Pressures of the Even-Numbered C 8 −C 18 Monocarboxylic Acids in J. Phys. Chem. A 112 (2008) 3959-3964, doi : 10.1021 / jp710586m .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-224.
Web links
- Center for Research on Lauric Oils, Inc. Archived from the original on February 1, 2009 ; accessed on October 27, 2015 .
